The first UV absorption band of l-tryptophan is not due to two simultaneous orthogonal electronic transitions differing in the dipole moment
Abstract
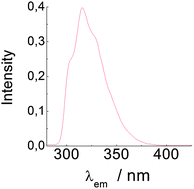
Based on UV/Vis spectroscopic evidence obtained in this work, the first band in the absorption spectrum of L-tryptophan is largely due to a single electronic transition from the ground state to the 1Lb excited state. However, emission spectra of this compound recorded at a variable temperature in ethanol, n-butanol and diethyl ether are structureless and considerably red-shifted at room temperature; also, lowering the temperature causes the emission to become structured and to undergo such a strong blue shift that it appears to be due to the 1Lb state of the compound. Based on these findings, the formation (from the excited 1Lb state) of the excited state responsible for the structureless, markedly red-shifted emission in L-tryptophan is strongly dependent not only on the viscosity of the medium, but also on its dipolarity.

 Please wait while we load your content...
Please wait while we load your content...