Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A systematic study on Pt based, subnanometer-sized alloy cluster catalysts for alkane dehydrogenation: effects of intermetallic interaction†
Andreas W.
Hauser‡
*a,
Paul R.
Horn
b,
Martin
Head-Gordon
b and
Alexis T.
Bell
*a
aDepartment of Chemical and Biomolecular Engineering, University of California, Berkeley, CA 94720-1462, USA. E-mail: andreas.w.hauser@gmail.com; alexbell@uclink.berkeley.edu
bDepartment of Chemistry, University of California, Berkeley, CA 94720-1462, USA
First published on 9th March 2016
Abstract
Platinum-based bimetallic nanoparticles are analyzed by the application of density functional theory to a series of tetrahedral Pt3X cluster models, with element X taken from the P-block, preferably group 14, or from the D-block around group 10. Almost identical cluster geometries allow a systematic investigation of electronic effects induced by different elements X. Choosing the propane-to-propene conversion as the desired dehydrogenation reaction, we provide estimates for the activity and selectivity of the various catalysts based on transition state theory. No significant Brønsted–Evans–Polanyi-relation could be found for the given reaction. A new descriptor, derived from an energy decomposition analysis, captures the effect of element X on the rate-determining step of the first hydrogen abstraction. Higher activities than obtained for pure Pt4 clusters are predicted for Pt alloys containing Ir, Sn, Ge and Si, with Pt3Ir showing particularly high selectivity.
1 Introduction
Platinum, in the form of supported nanoparticles, is one of the most suitable catalysts for the thermal dehydrogenation of light alkanes. The desired alkene products are building blocks for a wide array of commodity and specialty chemicals, or may be transformed into larger alkanes via follow-up oligomerization or metathesis reactions to serve as liquid fuel. Dehydrogenation is an attractive alternative to the traditional production of alkenes via steam cracking of petroleum-derived naphtha. Unpromoted Pt catalysts suffer from low alkene selectivity and rapid deactivation due to coking. Both undesired phenomena are mainly a consequence of alkene re-adsorption, which leads to further dehydrogenation and C–C bond breaking.1,2 Improvements of catalyst activity, selectivity, and stability can be achieved by alloying Pt with Sn and by adding hydrogen to the alkane feed.1,3–8 The positive effect of alloying with tin has been assumed to be a consequence of both the geometric as well as electronic modification.9–15 The presence of evenly distributed tin atoms on the surface hinders the formation of larger active sites, which suppresses undesired C–C bond breaking eventually leading to coking and catalyst deactivation.5,16–20 A recent experiment of our group on ethane dehydrogenation over PtSn nanoparticles (about 100 atoms, Pt/Sn ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) indicated beneficial electronic effects8 as they were suggested by earlier theoretical work on metal surfaces.12,13 Our own computational study on Pt4 and Pt3Sn tetramers predicted an improved dehydrogenation activity for the bimetallic nano cluster.21 The tin atom donates electron density to the active site of the catalyst, which reduces the energy barrier for the rate-determining step, the cleavage of the first C–H bond.
1) indicated beneficial electronic effects8 as they were suggested by earlier theoretical work on metal surfaces.12,13 Our own computational study on Pt4 and Pt3Sn tetramers predicted an improved dehydrogenation activity for the bimetallic nano cluster.21 The tin atom donates electron density to the active site of the catalyst, which reduces the energy barrier for the rate-determining step, the cleavage of the first C–H bond.
In this article we expand our previous work on platinum-based tetramers by a systematic quest for alternative bimetallic partner elements with similar or even better performance than the well-established Pt/Sn alloy. We compare the reaction pathways obtained from a density functional theory approach and apply ALMO-EDA (absolutely localized molecular orbital energy decomposition analysis) as well as NBO (natural bond orbital) theory to the crucial steps to gain further insights into the reaction mechanism.
2 Computational methods
We choose a representation by four atoms in a tetrahedral arrangement, which corresponds to the most stable structure among the Pt4 isomers.22–24 We then replace a single Pt atom by a different D-block or P-block metal and let the structure relax before propane is added to the system. Minima and intermediates occurring during the reaction with the alkane are obtained in fully unrestrained geometry optimizations. The freezing string method25,26 is applied to obtain initial guesses for the interconnecting transition states. This procedure is followed by a final localization based on an eigenvector-following approach.27 The transition states are then checked by frequency calculations proving their character as first-order saddle points on the potential energy surface. All calculations are performed with the Q-Chem program package.28 We further estimate Gibbs free energies within the harmonic oscillator approach at all relevant points of the reaction pathway, including also zero point energy corrections. All translational, rotational and vibrational degrees of freedom are taken into consideration. Hindered rotations are accounted for by applying the correction suggested by Grimme, which suggests a continuous interpolation between vibrational and rotational contributions to the entropy.29 A cutoff value of ω0 = 100 cm−1 was used for all calculations.Molecular geometries are obtained from density-functional-theory (DFT) in unconstrained optimizations, using the B3LYP functional30–33 together with the triple-zeta valence basis set of Weigend and Ahlrichs34,35 and the effective core potentials of the Stuttgart/Köln group36,37 for all metals. Effects of a van der Waals-correction38 are tested in a series of single point B3LYP-D3 calculations for all B3LYP-converged geometries. Details of the SCF convergence, DFT grid size information and thresholds of the geometry optimizations can be found in this footnote.39 Only Pt3Ir shows a strong deviation from the tetrahedral structure at the first C–H cleavage step with a slight tendency towards a planar geometry, where one of the edges between the active site and a Pt atom becomes elongated.
We further perform a natural bond order (NBO) analysis40,41 to investigate the relation between catalytic properties of each system and the shape of the molecular orbitals (MOs) involved. For a detailed analysis of donor–acceptor effects within the alloy clusters we apply ALMO-EDA,42,43 an energy decomposition scheme which splits intermolecular interaction energies into frozen orbital, polarization and charge transfer contributions.
3 Results and discussion
3.1 Reaction pathways for propane dehydrogenation over Pt4
We begin by revisiting the thermal dehydrogenation of propane over Pt4 and extend previous investigations of its potential energy surface21,44 by the exploration of alternative pathways. Results for electronic energies and Gibbs free energies at a rather mild reaction temperature of 400 °C are summarized in Table 1. The corresponding geometries and Gibbs free energy levels are illustrated in Fig. 1 and 2, respectively.| Reaction step | Label | ΔEa (kcal mol−1) | ΔGa (kcal mol−1) |
|---|---|---|---|
| a Relative to the energy of a pure Pt4 cluster and a propane molecule at infinite distance. b These values correspond to the electronic and Gibbs free energies for the gas phase reaction C3H8 ⇌ C3H6 + H2. | |||
| Propane adsorption at C2 | 1a | −12.22 | 13.04 |
| 1st C–H cleavage (TS) | 2a | −4.59 | 17.54 |
| Intermediate | 3a | −19.51 | 4.25 |
| H migration (TS) | 4a | −10.44 | 11.65 |
| Intermediate | 5a | −18.43 | 3.04 |
| 2nd C–H cleavage at C1 (TS) | 6a | −16.43 | 6.75 |
| Propane adsorption at C1, convex | 1b | −12.68 | 8.26 |
| 1st C–H cleavage (TS) | 2b | −5.61 | 16.14 |
| Intermediate | 3b | −19.56 | 3.87 |
| H migration (TS) | 4b | −9.32 | 14.09 |
| Intermediate | 5b | −18.11 | 3.86 |
| 2nd C–H cleavage at C2 (TS) | 6b | −16.37 | 6.66 |
| 2nd C–H cleavage at C1 (TS) | 1.49 | 22.11 | |
| Propane adsorption at C1, concave | 1c | −12.02 | 9.19 |
| 1st C–H cleavage (TS) | 2c | −5.31 | 17.05 |
| Propane adsorption, symmetric | 1d | −12.31 | 8.65 |
| Intermediate | 7 | −26.2 | −3.63 |
| H migration (TS) | 8 | −22.75 | −0.72 |
| Intermediate | 9 | −37.88 | −13.51 |
| 3rd C–H cleavage at C3 (TS) | 10a | −15.73 | 3.49 |
| 3rd C–H cleavage at C2 (TS) | 10b | −7.81 | 8.89 |
| 3rd C–H cleavage at C1 (TS) | 10c | −8.71 | 10.67 |
| Hydrogen desorption from Pt4–C3H6 | −8.98 | −8.69 | |
| Propene desorption from Pt4–H–H | 3.27 | −2.63 | |
| Hydrogen and propene desorptionb | 35.76 | 8.86 | |
| C–C breaking in Pt4–C3H8 | 17.36 | 41.33 | |
| C–C breaking in H–Pt4–CH3–CH–CH3 | 20.37 | 41.4 | |
| C–C breaking in H–Pt4–CH3–CH2–CH2 | 13.53 | 36.1 | |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C breaking in H–H–Pt4–C3H6 C breaking in H–H–Pt4–C3H6 |
34.36 | 49.49 | |
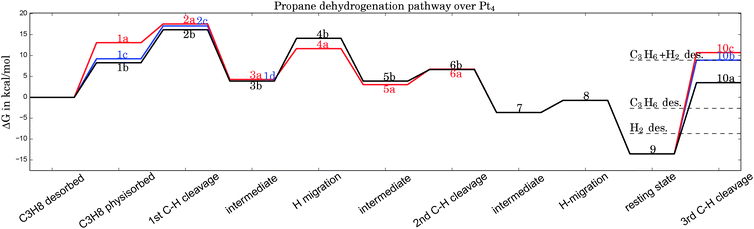 | ||
| Fig. 2 Gibbs free energy diagram of possible dehydrogenation pathways, calculated at 400 °C. The first hydrogen abstraction is the rate-determining step and is most likely to happen at C1, corresponding to geometry 2b in Fig. 1. Desorption energies are plotted as dashed lines. | ||
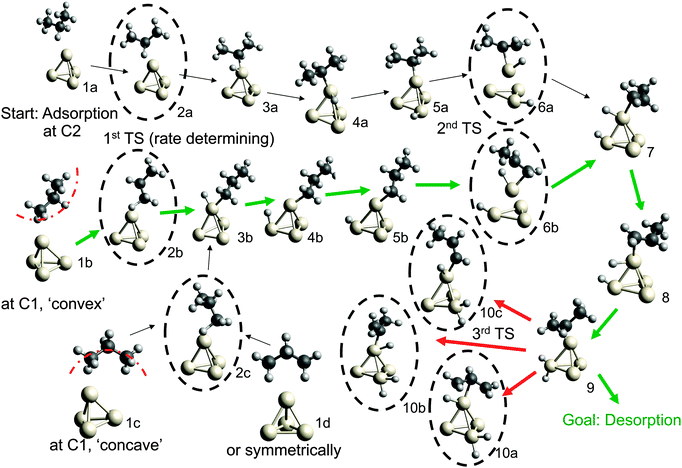
Possible adsorption scenarios involve either C2–H–Pt (see geometry 1a) or C1–H–Pt type interactions. In the latter case, three adsorption minima could be determined, where the propane is either bent towards the cluster (concave, 1c), bent off the cluster (convex, 1b), or in a highly symmetric arrangement 1d of C2v symmetry. The strong C–H σ bonds, which are essentially nonpolar and therefore weak donors, act as ligands in the unusual high-symmetry arrangement.45 In general, the direct observation of such σ-dihydrogen species is problematic due to the instability of the complexes formed, but could give insights into early steps of metal-mediated H–H or C–H cleavage. Recently, a relatively long-lived rhodium(I) σ-methane complex of similar geometry46 (in solution) was characterized by NMR. We mention this finding since examples of saturated hydrocarbons within the coordination sphere of a metal center in the solid state are extremely rare.47–49
The adsorption step is directly followed by hydrogen abstraction, leading to an intermediate where the remaining alkyl group and the H atom are attached to the same corner (3a or 3b of Fig. 1). Previous studies have focused on a reaction mechanism in which C–H bond breaking at the C2 atom (2a) is followed by hydrogen abstraction from C1 (6a).21,44 However, two alternative pathways, where the C–H bond cleavage takes place at C1 before C2 (with the propane molecule in either the ‘convex’ 2b or ‘concave’ 2c arrangement), show slightly lower barriers for the rate-determining transition state as can be seen in Table 1. After the migration of the hydrogen atom to a different corner, the second hydrogen abstraction takes place, preferably at C2 if the first abstraction took place at C1 and vice versa. Alternative pathways, such as a repeated hydrogen abstraction from the same carbon atom or the breaking of C–C bonds are omitted from the figure due to the higher barriers involved but are listed in the table. Both pathways merge after the second hydrogen abstraction. Further H migration (7, 8) leads to the minimum energy configuration 9 with two hydrogen atoms attached to the same corner. After this step the reaction path forks to either propene desorption or further dehydrogenation via a third C–H cleavage step (10a, 10b or 10c).
From Table 1 it can be seen that at higher temperatures the desorption energy for propene decreases under the barrier for an undesired further dehydrogenation step, which explains the high selectivity towards the product alkene.21 This energy difference between the true barrier for a third H abstraction and the energy needed to desorb the products is an important property as it affects the selectivity. The activity of the catalyst, on the other hand, is dictated by the highest transition state along the free energy reaction pathway, corresponding to the first C–H cleavage step. The energies for this particular reaction step in all pathways (2a, 2b, 2c) differ by about 1 kcal mol−1, with the option of an initial abstraction from C1 being slightly preferred. The energetic proximity of the first transition states makes it necessary to compute all three of them in the following comparison of bimetallic catalysts.
For the sake of readability, the detailed discussion of the reaction pathway after inclusion of an ad hoc van der Waals correction (B3LYP-D3) has been shifted to the ESI.† We note that this measure enhances the adsorption energies by about 5 kcal mol−1 on average, but it has a negligible effect on the relative energies of the pathways shown in Fig. 2, and thus it does not affect the trends discussed in the next section.
3.2 Bimetallic variations of the tetrahedral motif: Pt3X clusters
We broaden our analysis of the dehydrogenation reaction by the introduction of a second metal to our tetrahedral nanoparticle. A common ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 has been chosen for the bimetallic systems,50–53 which is easily achieved by a single atom replacement. Initial guesses for the bimetallic geometries of the various Pt3X systems are obtained from the relevant stationary points of the Pt4 reaction pathway by replacing one of the three Pt atoms which do not directly participate in the propane adsorption. For X we choose Pt,44 Pd,54,55 Sn,8,56,57 Ge,58 Si, In,59 Ga,52 Au,60,61 Ag,62,63 Cu,64 Ir.65,66 For the sake of a direct comparison we treat Pt atoms as the only active sites of the catalyst. This simplification is supported by a Mulliken charge analysis of the pure clusters, which shows a positive charge on the replacement atom in all cases.67,68 The spin multiplicities of the different alloys vary with element X and lie between singlet and quartet for the given selection.69 In contrast to the previous section we will focus now only on a subset of relevant geometries for the sake of readability. Furthermore, we ignore the presence of any hydrogen atoms on the catalyst from previous dehydrogenation steps.70 This is done for two reasons. First, the amount of hydrogen on the catalyst during the reaction depends not only on the current reaction step but also on the partial pressure of hydrogen21 and on the interplay between the nanoparticle and its support. Both aspects are worthy of study on their own but lie beyond the scope of this article. Second, the presence of hydrogen on the catalyst introduces a bias when comparing pathways between different catalysts due to numerous hydride isomers of similar energy, and it complicates the potential energy surfaces considerably due to additional transition states and local minima that correspond to hydrogen migration. Therefore, we reduce our analysis to the following geometries: the clean catalyst, propane adsorption, propene adsorption, and the transition states describing first, second and third C–H bond cleavage, together with their corresponding precursor intermediates. Pairs of the latter, i.e. transition states and their preceding intermediates, allow an unbiased comparison of true barriers along the reaction pathway.
3 has been chosen for the bimetallic systems,50–53 which is easily achieved by a single atom replacement. Initial guesses for the bimetallic geometries of the various Pt3X systems are obtained from the relevant stationary points of the Pt4 reaction pathway by replacing one of the three Pt atoms which do not directly participate in the propane adsorption. For X we choose Pt,44 Pd,54,55 Sn,8,56,57 Ge,58 Si, In,59 Ga,52 Au,60,61 Ag,62,63 Cu,64 Ir.65,66 For the sake of a direct comparison we treat Pt atoms as the only active sites of the catalyst. This simplification is supported by a Mulliken charge analysis of the pure clusters, which shows a positive charge on the replacement atom in all cases.67,68 The spin multiplicities of the different alloys vary with element X and lie between singlet and quartet for the given selection.69 In contrast to the previous section we will focus now only on a subset of relevant geometries for the sake of readability. Furthermore, we ignore the presence of any hydrogen atoms on the catalyst from previous dehydrogenation steps.70 This is done for two reasons. First, the amount of hydrogen on the catalyst during the reaction depends not only on the current reaction step but also on the partial pressure of hydrogen21 and on the interplay between the nanoparticle and its support. Both aspects are worthy of study on their own but lie beyond the scope of this article. Second, the presence of hydrogen on the catalyst introduces a bias when comparing pathways between different catalysts due to numerous hydride isomers of similar energy, and it complicates the potential energy surfaces considerably due to additional transition states and local minima that correspond to hydrogen migration. Therefore, we reduce our analysis to the following geometries: the clean catalyst, propane adsorption, propene adsorption, and the transition states describing first, second and third C–H bond cleavage, together with their corresponding precursor intermediates. Pairs of the latter, i.e. transition states and their preceding intermediates, allow an unbiased comparison of true barriers along the reaction pathway.
Fig. 3 illustrates the adsorption energies for propane onto the various catalysts. The fluctuation with catalyst type is only moderate and lies within a range of ±2.5 kcal mol−1 around the average. The catalysts on the x-axis are sorted with respect to their apparent barriers for the rate-determining, first H-abstraction step at 0 Kelvin. Therefore, the x-axis can be read as a ranking of catalyst activity, starting with the best catalyst on the left end. As can be seen in Fig. 3, there is no clear correlation of activity and propane adsorption. At the high temperatures required for the endothermic reaction of propane dehydrogenation (ΔH = 20.1 kcal mol−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71,72), the change in Gibbs energies upon adsorption is positive. Based on DFT thermochemistry data we estimate that propane desorption becomes spontaneous at temperatures above 200 °C for most catalysts. Note that the replacement of one Pt atom by any of the suggested elements X leads to a slightly enhanced physisorption of propane at the Pt sites. In bulk alloys these small electronic electronic effects tend to be overruled by geometric and relaxation effects. A comparable DFT study of propane adsorption onto Pt(111) and Pt3Sn(111) surfaces shows a slightly reduced physisorption for the alloy in comparison to the pure Pt surface (ΔE = 0.9 kcal mol−1).73
71,72), the change in Gibbs energies upon adsorption is positive. Based on DFT thermochemistry data we estimate that propane desorption becomes spontaneous at temperatures above 200 °C for most catalysts. Note that the replacement of one Pt atom by any of the suggested elements X leads to a slightly enhanced physisorption of propane at the Pt sites. In bulk alloys these small electronic electronic effects tend to be overruled by geometric and relaxation effects. A comparable DFT study of propane adsorption onto Pt(111) and Pt3Sn(111) surfaces shows a slightly reduced physisorption for the alloy in comparison to the pure Pt surface (ΔE = 0.9 kcal mol−1).73
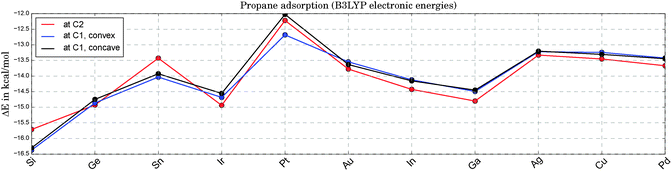 | ||
| Fig. 3 Electronic energies for the adsorption of propane onto Pt3X (see 1a, 1b and 1c in Fig. 1) at zero Kelvin. Due to the weak adsorption the Gibbs free energies are all positive at experimentally relevant temperatures, indicating a negligible coverage of the catalyst with physisorbed propane. | ||
For the sake of completeness we also tested the X sites for their adsorption qualities. In all cases, the propane adsorption on atom X is either weaker than on Pt (by 2.9 kcal mol−1 on average) or an adsorption minimum could not be found (Ga, Ge, In, Sn). This agrees well with the initial finding of positive Mulliken charges at X for all Pt3X systems under consideration.
In Fig. 4 we compare the apparent barriers for the first C–H bond cleavage. This step being the bottleneck of the overall reaction pathway, we plot ΔG, the change in Gibbs free energy, with G set to zero for propane at an infinite distance from the catalyst. We note that Ir shows a distortion from the perfect tetrahedral shape during C–H bond breakage, which introduces a slight bias in the overall comparison due to geometry effects. The distance from the active center to one Pt atom is extended by about 60%, leading to a geometry between square planar and tetrahedral.
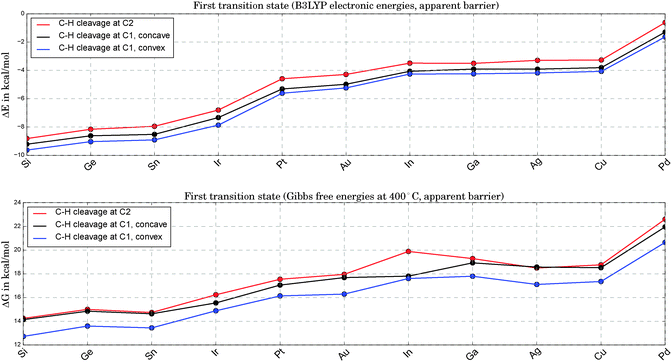 | ||
| Fig. 4 Comparison of electronic energy barriers (upper graph) and apparent Gibbs free energy barriers at 400 °C (lower graph) for the first TS states of Pt3X (see 2a, 2b and 2c in Fig. 1). For all alloys the lowest of these options for the first C–H bond cleavage corresponds to the rate-determining step. | ||
Energies for the second hydrogen abstraction are not discussed here but can be found in the supporting material. There is no clear trend detectable in the barrier for this step. In general, the differences between alloys are marginal.
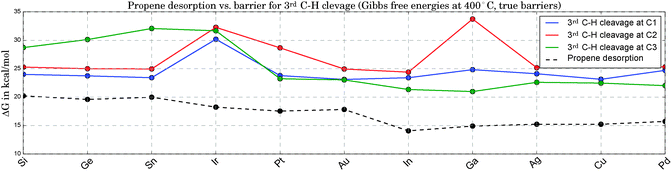
Fig. 5 compares the true barriers for the third hydrogen abstraction from C1, C2 or C3, to the desorption energy for propene. Again, we provide ΔG values at 400 °C. At this high temperature, due to the gain in entropy upon desorption, the production of propene becomes competitive with the undesired pathways of continued dehydrogenation. The latter steps eventually lead to methane and the formation of coke on the catalyst. The direct comparison of the ΔG values for propene desorption to the energetically lowest option for the third C–H bond cleavage allows an estimate to be made of the selectivity towards propene. The larger the difference between the lowest barrier and the desorption energy, the better. Propene desorption is preferred for all catalysts in this study at B3LYP level of theory,74 but this difference shows large fluctuations. It is smallest for the three most active catalysts and larger for the least active clusters, but a clear trend is not apparent. Pt3Ir seems to be a special case due to its comparably high barriers for the 3rd C–H cleavage, which makes it particularly interesting for future experimental investigations due to the expected higher selectivity. However, this discrepancy might also be related to the geometric distortions observed for Pt3Ir.
3.3 Descriptors for catalytic activity
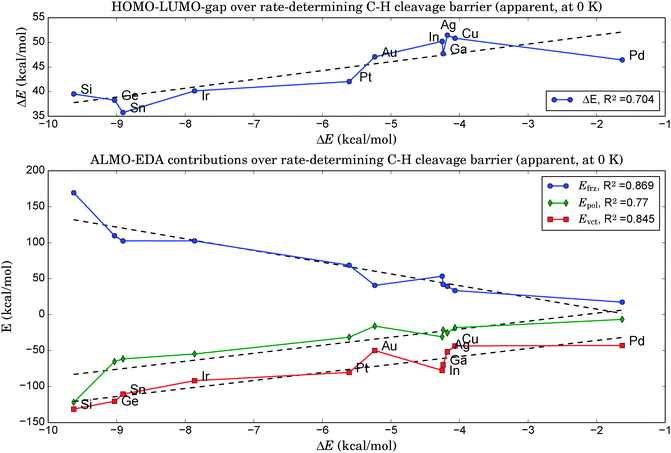
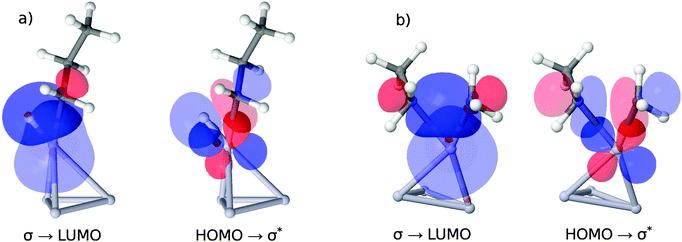
As illustrated in the upper panel of Fig. 6, a property of the clean catalyst, namely its HOMO–LUMO gap, is correlated with activity (R2 = 0.71). The smaller the gap, the lower is the barrier of the rate-determining C–H cleavage step. Retrieving this information is significantly less laborious than a full evaluation of the dehydrogenation reaction for a given catalyst. It only necessitates the optimization of the pure cluster geometry as a prerequisite.The four orbitals involved in C–H cleavage are plotted in panel (a) of Fig. 7, given the example of Pt4. The first interaction (left) describes a charge transfer from the σ-bond orbital of the activated C–H bond to an unoccupied, almost spherical and rather diffuse d-orbital of the nearest Pt atom. The second interaction describes an electron back-donation from an occupied d-orbital of the same Pt atom (well defined, two nodal planes) at the active site into the unoccupied anti-bonding σ* orbital of the activated C–H bond, which shows the typical extra nodal plane. Therefore, within the NBO picture, the C–H bond cleavage is described as the usual transfer of electron density from the bonding into the anti-bonding orbital, mediated via the d-orbitals of the catalyst. What links this process to a property of the clean catalyst is that the two NBOs on the catalyst fragment correspond quite closely to the HOMO and the LUMO of the cluster in the canonical orbital set, as can be deduced from a comparison of their coefficients in the basis of atomic orbitals. However, while this proves the relevance of these orbitals for C–H bond activation, it does not explain the dependence of the activity on their energy gap. A possible explanation involves the increasing flexibility (i.e. greater polarizability and greater ability to participate in CT interactions) of the electron density with decreasing HOMO–LUMO gap, commonly referred to as chemical ‘softness’. On the other hand, this argument predicts highest activities for bulk alloys with zero band gaps. We therefore suggest that an optimum catalyst combines chemical softness with a sterically suitable nodal structure of its valence orbitals which allows a precise transfer of electron density in favor of the geometric arrangement after the hydrogen abstraction.
In panel (b) of Fig. 7 we apply the same NBO analysis to the situation of C–C bond breaking catalyzed by Pt4 for a direct comparison. Again, we find the HOMO and LUMO orbital of the clean catalyst involved in a charge transfer process, indicating a similar dependence of the energetic barrier for C–C cleavage on the HOMO–LUMO gap. However, as can be derived from the energies in Table 1, this dependence is much less pronounced, and the apparent barrier heights are much higher. The higher energies are a consequence of the suboptimal arrangement of the orbitals during C–C bond breaking. A comparison of panels (a) and (b) shows that in the case of C–C breaking two directed sp3 lobes of the remaining methyl- and ethyl groups need to overlap with the d-orbital on the active metal site, while for C–H breaking only the sp3 orbital on the propyl-group has similar spatial demands. The undirected s-orbital on the H atom retains good overlap throughout the geometric rearrangement, which explains, together with the smaller angle between the lobes of the sp3 and d orbital in case (a), the lower energetic costs for H abstraction.
From a microscopic point of view, relations of the latter type indicate that the transition state must be ‘late’ in the sense of its geometry being close to that of the subsequent intermediate. Fortunately, this is true for the rate-determining step of the given reaction. Therefore, it should be possible to relate the adsorption energies of the chemisorbed products right after dissociation, here a propyl-group and a single hydrogen atom, to the barrier height for C–H bond breaking. Note that the chemisorption of the intermediates after hydrogen cleavage, not the physisorption of propane, can be a potential descriptor, since the latter is based on a different (i.e. van der Waals)-type of binding. Following ref. 84, we first assume that the chemisorption of a single H atom does not vary too strongly for the chosen catalysts. In this case, the BEP relation should also hold for just the alkyl fragment. A further simplification made by choosing a methyl group as smallest representative with the same valency leads to the relation shown in Fig. 8a. However, this graph shows a rather weak correlation (R2 = 0.422), indicating that a simple mapping of kinetics onto thermodynamics is not applicable for the reaction of interest here. This is somewhat surprising, since for all catalysts tested the typical C–H bond length at the transition state lies around 1.49 Å, which suggests an almost broken bond and a close proximity to the subsequent intermediate state with the cleaved H atom chemisorbed at the same site.
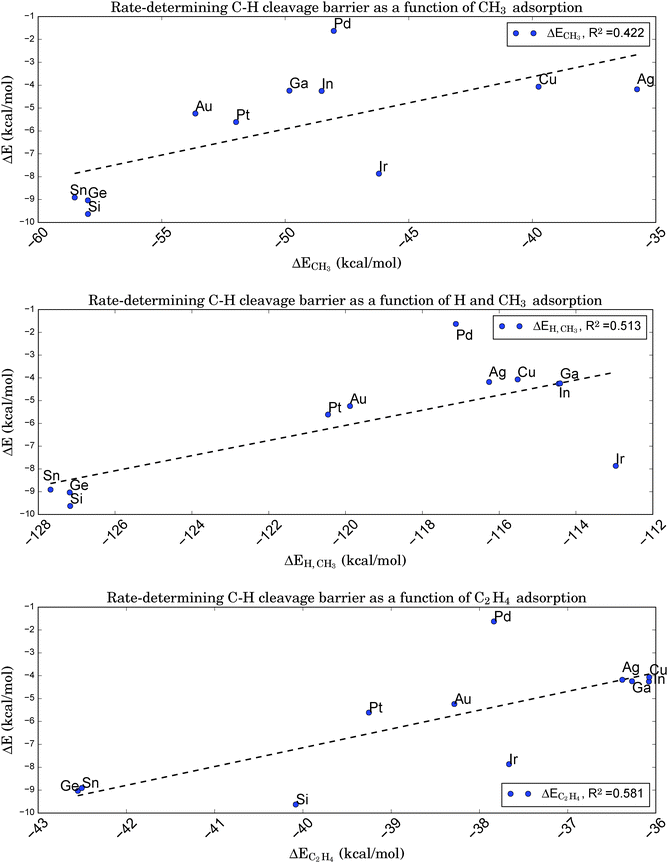 | ||
| Fig. 8 Brønsted–Evans–Polanyi relation between the adsorption energies of a CH3 fragment (upper graph), a CH3 fragment and a single H atom (middle graph), or a C2H4 molecule (lower graph) onto Pt3X and the rate-determining first C–H cleavage barrier for the conversion of propane to propene. Despite the ‘late’ character of the corresponding transition state the assumed correlation to the absorption of the alkyl fragment is very weak. The correlation is slightly improved if a simultaneous adsorption of H and CH3 at the same site is considered. Surprisingly, the adsorption of C2H4, the smallest alkene representative, is still better correlated, although the correlation is significantly poorer than was obtained in Fig. 6 based on electronic properties of the clean catalyst. | ||
Adding the chemisorption energy for atomic hydrogen to the energy for methyl chemisorption and comparing this total energy to the barrier height for C–H cleavage reduces the BEP correlation even further (R2 = 0.228), since variations in ΔEH turn out to be of the same magnitude as ΔECH3, but are completely uncorrelated to the C–H cleavage barriers (R2 = 0.014). However, a direct comparison of energies for the simultaneous adsorption of CH3 and a single H atom at the same site shows a slightly improved correlation (R2 = 0.513, see Fig. 8b) due to the inclusion of adsorbate–adsorbate interactions.
In a last attempt to derive a suitable BEP relation we compare the barrier height for C–H cleavage to the adsorption of an alkene. We pick ethene as the simplest representative of a molecule with a C–C double bond and obtain the relation shown in Fig. 8c. Interestingly, this graph shows a slightly higher correlation (R2 = 0.581) than that based on CH3 fragment energies, despite the larger ‘distance’ of the simulated geometry of alkene adsorption from the relevant transition state with respect to the reaction coordinate.
4 Conclusion
The dehydrogenation of propene over platinum-based, bimetallic nanoparticles was studied via B3LYP and B3LYP-D3 calculations on Pt3X model systems. Crucial steps of the reaction pathway were identified in unconstrained geometry optimizations and transition state searches. Gibbs energies were obtained from frequency calculations and corrected for hindered rotations. We draw the following conclusions:1. The physisorption of propane on bimetallic Pt3X clusters is only weakly dependent on the replacement atom X, inducing a change of electronic energies on the order of about 2.5 kcal mol−1. ΔG, the change of Gibbs energy upon propane uptake, becomes positive at higher temperatures. The average value at 400 °C lies at 8.1 kcal mol−1 (4.2 kcal mol−1 with D3 correction).
2. The first C–H cleavage is the rate-determining step for all catalysts of the test set, but its barrier varies significantly with element X. At 400 °C we obtain ΔG values between 12 and 21 kcal mol−1 (between 8 and 16 kcal mol−1 with D3 correction). Similar or slightly higher activities than calculated for Pt4 are predicted for Pt3Si, Pt3Ge, Pt3Sn and Pt3Ir.
3. The catalytic activity is correlated with the HOMO–LUMO gap of the clean catalyst. Both orbitals participate in the activation of the C–H bond at the relevant transition state. The σ-bond of the propane molecule donates charge into a diffuse d-orbital at the active site, which corresponds to the LUMO of the clean catalyst. The HOMO of the latter donates charge into the corresponding anti bonding orbital, which stabilizes the new geometry obtained after C–H bond cleavage.
4. Smaller HOMO–LUMO gaps indicate higher activity due to the increased flexibility of the valence electron at the active site. An NBO analysis reveals that the d-orbital shape of the HOMO with its pronounced nodal features better facilitates the breaking of a C–H bond than of a C–C bond due to better overlap with the C–H anti-binding orbital.
5. A very effective and computationally cheap descriptor for the catalytic activity could be found via an energy decomposition analysis at the equilibrium geometries of the clean catalysts by treating the dopant atom X as one fragment and the remaining catalyst Pt3 as the other. The higher the energy contributions of frozen orbital interactions, Efrz, the lower was the rate-determining step in the follow-up evaluation of the propane dehydrogenation pathways (R2 = 0.87).
6. No significant Brønsted–Evans–Polanyi-relation could be found for the given reaction. The simultaneous chemisorption of atomic hydrogen and a methyl group, the smallest representative with the same valency as the corresponding reaction intermediate, on the same Pt site, is only weakly correlated (R2 = 0.51) to the barrier height of the rate-determining C–H cleavage step, despite the ‘late’ character of the relevant transition state.
7. The experimentally observed high selectivity towards propene agrees well with the finding that direct C–C bond breaking, a typical, preliminary step towards coking, is less likely due to higher barriers compared to C–H bond breaking. However, transition states that lead to undesired, continued dehydrogenation of the reactant compete with the desorption of the products after the second H cleavage step. At higher temperatures, desorption becomes more likely due to the gain in entropy, explaining an overall preference towards the desired product. Pt3Ir seems to be exceptionally selective due to the higher barriers for continued C–H abstraction, but a slight bias due to geometric effects could not be excluded in this case.
Acknowledgements
This research was supported by the XC2 program funded by BP. Calculations were performed on a cluster provided by the UC Berkeley College of Chemistry through the National Science Foundation (NSF) (Grant CHE-1048789). Development of EDA methodology and application to this system was supported by a grant from the National Science Foundation (CHE-1363342). AWH thanks Dennis Salahub for helpful discussions at the CMMSE conference held in Rota, Spain.References
- V. Galvita, G. Siddiqi, P. Sun and A. T. Bell, J. Catal., 2010, 271, 209–219 CrossRef CAS.
- A. Virnovskaia, E. Rytter and U. Olsbye, Ind. Eng. Chem. Res., 2008, 47, 7167–7177 CrossRef CAS.
- O. A. Bariås, A. Holmen and E. A. Blekkan, J. Catal., 1996, 158, 1–12 CrossRef.
- F. Delbecq and P. Sautet, J. Catal., 2003, 220, 115–126 CrossRef CAS.
- M. S. Kumar, D. Chen, A. Holmen and J. C. Walmsley, Catal. Today, 2009, 142, 17–23 CrossRef.
- S. Slimane Laref, F. Delbecq and D. Loffreda, J. Catal., 2009, 265, 35–42 CrossRef.
- F. Vigné, J. Haubrich, D. Loffreda, P. Sautet and F. Delbecq, J. Catal., 2010, 275, 129–139 CrossRef.
- J. Wu, Z. Peng and A. T. Bell, J. Catal., 2014, 311, 161–168 CrossRef CAS.
- J. Shen, J. M. Hill, R. M. Watwe, B. E. Spiewak and J. A. Dumesic, J. Phys. Chem. B, 1999, 103, 3923–3934 CrossRef CAS.
- R. M. Watwe, R. D. Cortright, M. Mavrikakis, J. K. Norskov and J. A. Dumesic, J. Chem. Phys., 2001, 114, 4663–4668 CrossRef CAS.
- E. Janin, H. von Schenck, S. Ringler, J. Weissenrieder, T. Åkermark and M. Göthelid, J. Catal., 2003, 215, 245–253 CrossRef CAS.
- R. Alcala, J. W. Shabaker, G. W. Huber, M. A. Sanchez-Castillo and J. A. Dumesic, J. Phys. Chem. B, 2005, 109, 2074–2085 CrossRef CAS PubMed.
- J. Essen, J. Haubrich, C. Becker and K. Wandelt, Surf. Sci., 2007, 601, 3472–3480 CrossRef CAS.
- A. Virnovskaia, S. Morandi, E. Rytter, G. Ghiotti and U. Olsbye, J. Phys. Chem. C, 2007, 111, 14732–14742 CAS.
- A. Virnovskaia, S. Jørgensen, J. Hafizovic, O. Prytz, E. Kleimenov, M. Hävecker, H. Bluhm, A. Knop-Gericke, R. Schlögl and U. Olsbye, Surf. Sci., 2007, 601, 30–43 CrossRef CAS.
- Y.-L. Tsai and B. E. Koel, J. Phys. Chem. B, 1997, 101, 2895–2906 CrossRef CAS.
- G. J. Siri, J. M. Ramallo-López, M. L. Casella, J. L. Fierro, F. G. Requejo and O. A. Ferretti, Appl. Catal., A, 2005, 278, 239–249 CrossRef CAS.
- M. P. Lobera, C. Téllez, J. Herguido and M. Menéndez, Appl. Catal., A, 2008, 349, 156–164 CrossRef CAS.
- Z. Nawaz, X. Tang, Q. Zhang, D. Wang and W. Fei, Catal. Commun., 2009, 10, 1925–1930 CrossRef CAS.
- S. Pisduangdaw, J. Panpranot, C. Methastidsook, C. Chaisuk, K. Faungnawakij, P. Praserthdam and O. Mekasuwandumrong, Appl. Catal., A, 2009, 370, 1–6 CrossRef CAS.
- A. W. Hauser, J. Gomes, M. Bajdich, M. Head-Gordon and A. T. Bell, Phys. Chem. Chem. Phys., 2013, 15, 20727–20734 RSC.
- L. Xiao and L. Wang, J. Phys. Chem. A, 2004, 108, 8605–8614 CrossRef CAS.
- L. Xiao and L. Wang, J. Phys. Chem. B, 2007, 111, 1657–1663 CrossRef CAS PubMed.
- J. Kua and W. A. Goddard, J. Phys. Chem. B, 1998, 102, 9481–9491 CrossRef CAS.
- S. Mallikarjun Sharada, P. M. Zimmerman, A. T. Bell and M. Head-Gordon, J. Chem. Theory Comput., 2012, 8, 5166–5174 CrossRef CAS PubMed.
- A. Behn, P. M. Zimmerman, A. T. Bell and M. Head-Gordon, J. Chem. Phys., 2011, 135, 224108 CrossRef PubMed.
- J. Baker, J. Comput. Chem., 1986, 7, 385–395 CrossRef CAS.
- Y. Shao, Z. Gan, E. Epifanovsky, A. T. Gilbert, M. Wormit, J. Kussmann, A. W. Lange, A. Behn, J. Deng, X. Feng, D. Ghosh, M. Goldey, P. R. Horn, L. D. Jacobson, I. Kaliman, R. Z. Khaliullin, T. Kuś, A. Landau, J. Liu, E. I. Proynov, Y. M. Rhee, R. M. Richard, M. A. Rohrdanz, R. P. Steele, E. J. Sundstrom, H. L. Woodcock, P. M. Zimmerman, D. Zuev, B. Albrecht, E. Alguire, B. Austin, G. J. O. Beran, Y. A. Bernard, E. Berquist, K. Brandhorst, K. B. Bravaya, S. T. Brown, D. Casanova, C.-M. Chang, Y. Chen, S. H. Chien, K. D. Closser, D. L. Crittenden, M. Diedenhofen, R. A. DiStasio, H. Do, A. D. Dutoi, R. G. Edgar, S. Fatehi, L. Fusti-Molnar, A. Ghysels, A. Golubeva-Zadorozhnaya, J. Gomes, M. W. Hanson-Heine, P. H. Harbach, A. W. Hauser, E. G. Hohenstein, Z. C. Holden, T.-C. Jagau, H. Ji, B. Kaduk, K. Khistyaev, J. Kim, J. Kim, R. A. King, P. Klunzinger, D. Kosenkov, T. Kowalczyk, C. M. Krauter, K. U. Lao, A. Laurent, K. V. Lawler, S. V. Levchenko, C. Y. Lin, F. Liu, E. Livshits, R. C. Lochan, A. Luenser, P. Manohar, S. F. Manzer, S.-P. Mao, N. Mardirossian, A. V. Marenich, S. A. Maurer, N. J. Mayhall, E. Neuscamman, C. M. Oana, R. Olivares-Amaya, D. P. ONeill, J. A. Parkhill, T. M. Perrine, R. Peverati, A. Prociuk, D. R. Rehn, E. Rosta, N. J. Russ, S. M. Sharada, S. Sharma, D. W. Small, A. Sodt, T. Stein, D. Stück, Y.-C. Su, A. J. Thom, T. Tsuchimochi, V. Vanovschi, L. Vogt, O. Vydrov, T. Wang, M. A. Watson, J. Wenzel, A. White, C. F. Williams, J. Yang, S. Yeganeh, S. R. Yost, Z.-Q. You, I. Y. Zhang, X. Zhang, Y. Zhao, B. R. Brooks, G. K. Chan, D. M. Chipman, C. J. Cramer, W. A. Goddard, M. S. Gordon, W. J. Hehre, A. Klamt, H. F. Schaefer, M. W. Schmidt, C. D. Sherrill, D. G. Truhlar, A. Warshel, X. Xu, A. Aspuru-Guzik, R. Baer, A. T. Bell, N. A. Besley, J.-D. Chai, A. Dreuw, B. D. Dunietz, T. R. Furlani, S. R. Gwaltney, C.-P. Hsu, Y. Jung, J. Kong, D. S. Lambrecht, W. Liang, C. Ochsenfeld, V. A. Rassolov, L. V. Slipchenko, J. E. Subotnik, T. Van Voorhis, J. M. Herbert, A. I. Krylov, P. M. Gill and M. Head-Gordon, Mol. Phys., 2015, 113, 184–215 CrossRef CAS.
- S. Grimme, Chem. – Eur. J., 2012, 18, 9955–9964 CrossRef CAS PubMed.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372–1377 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- f-functions have been removed from all basis sets for performance reasons.
- D. Andrae, U. Häußermann, M. Dolg, H. Stoll and H. Preuß, Theor. Chim. Acta, 1990, 77, 123–141 CrossRef CAS.
- B. Metz, H. Stoll and M. Dolg, J. Chem. Phys., 2000, 113, 2563–2569 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- The SCF convergence in all optimizations is set to 10−9 Hartree, for the DFT grid size we choose 75 radial and 302 Lebedev angular points. The convergence thresholds for the maximum gradient component and for the energy difference of successive optimization steps are set to 3 × 10−5 and 4 × 10−7 Hartree, respectively.
- E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales and F. Weinhold, NBO 5.0, Theoretical Chemistry Institute, University of Wisconsin, Madison, 2001 Search PubMed.
- E. D. Glendening, C. R. Landis and F. Weinhold, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 1–42 CrossRef CAS.
- R. Z. Khaliullin, E. A. Cobar, R. C. Lochan, A. T. Bell and M. Head-Gordon, J. Phys. Chem. A, 2007, 111, 8753–8765 CrossRef CAS PubMed.
- P. R. Horn, E. J. Sundstrom, T. A. Baker and M. Head-Gordon, J. Chem. Phys., 2013, 138, 134119 CrossRef PubMed.
- S. Vajda, M. J. Pellin, J. P. Greeley, C. L. Marshall, L. A. Curtiss, G. A. Ballentine, J. W. Elam, S. Catillon-Mucherie, P. C. Redfern, F. Mehmood and P. Zapol, Nat. Mater., 2009, 8, 213–216 CrossRef CAS PubMed.
- G. J. Kubas, Metal Dihydrogen and σ-Bond Complexes, Kluwer Academic, New York, 2001 Search PubMed.
- W. H. Bernskoetter, C. K. Schauer, K. I. Goldberg and M. Brookhart, Science, 2009, 326, 553–556 CrossRef CAS PubMed.
- D. R. Evans, T. Drovetskaya, R. Bau, C. A. Reed and P. D. W. Boyd, J. Am. Chem. Soc., 1997, 119, 3633–3634 CrossRef CAS.
- I. Castro-Rodriguez, H. Nakai, P. Gantzel, L. N. Zakharov, A. L. Rheingold and K. Meyer, J. Am. Chem. Soc., 2003, 125, 15734–15735 CrossRef CAS PubMed.
- S. D. Pike, A. L. Thompson, A. G. Algarra, D. C. Apperley, S. A. Macgregor and A. S. Weller, Science, 2012, 337, 1648–1651 CrossRef CAS PubMed.
- V. R. Stamenkovic, B. S. Mun, M. Arenz, K. J. J. Mayrhofer, C. A. Lucas, G. Wang, P. N. Ross and N. M. Markovic, Nat. Mater., 2007, 6, 241–247 CrossRef CAS PubMed.
- N. S. Porter, H. Wu, Z. Quan and J. Fang, Acc. Chem. Res., 2013, 46, 1867–1877 CrossRef CAS PubMed.
- Y. Xu, A. C. Lausche, S. Wang, T. S. Khan, F. Abild-Pedersen, F. Studt, J. K. Nørskov and T. Bligaard, New J. Phys., 2013, 15, 125021 CrossRef.
- M. Melník, P. Mikuš and C. E. Holloway, Cent. Eur. J. Chem., 2013, 11, 1902–1953 Search PubMed.
- Z.-F. Xu and Y. Wang, J. Phys. Chem. C, 2011, 115, 20565–20571 CAS.
- J. Wu, L. Qi, H. You, A. Gross, J. Li and H. Yang, J. Am. Chem. Soc., 2012, 134, 11880–11883 CrossRef CAS PubMed.
- Y. Liu, D. Li, V. R. Stamenkovic, S. Soled, J. D. Henao and S. Sun, ACS Catal., 2011, 1, 1719–1723 CrossRef CAS.
- X. Wang, L. Altmann, J. Stöver, V. Zielasek, M. Bäumer, K. Al-Shamery, H. Borchert, J. Parisi and J. Kolny-Olesiak, Chem. Mater., 2013, 25, 1400–1407 CrossRef CAS.
- M. Tanabe, K. Tanaka, S. Omine and K. Osakada, Chem. Commun., 2014, 50, 6839–6842 RSC.
- P. Sun, G. Siddiqi, W. C. Vining, M. Chi and A. T. Bell, J. Catal., 2011, 282, 165–174 CrossRef CAS.
- K.-S. Lee, H.-Y. Park, H. C. Ham, S. J. Yoo, H. J. Kim, E. Cho, A. Manthiram and J. H. Jang, J. Phys. Chem. C, 2013, 117, 9164–9170 CAS.
- S.-Y. Lee, N. Jung, J. Cho, H.-Y. Park, J. Ryu, I. Jang, H.-J. Kim, E. Cho, Y.-H. Park, H. C. Ham, J. H. Jang and S. J. Yoo, ACS Catal., 2014, 4, 2402–2408 CrossRef CAS.
- A. D. Burrows, J. G. Jeffrey, J. C. Machell and D. P. Mingos, J. Organomet. Chem., 1991, 406, 399–408 CrossRef CAS.
- S. Bhaduri, K. Sharma, P. G. Jones and C. F. Erdbrügger, J. Organomet. Chem., 1987, 326, C46–C48 CrossRef CAS.
- B. Y. Xia, H. B. Wu, X. Wang and X. W. D. Lou, J. Am. Chem. Soc., 2012, 134, 13934–13937 CrossRef CAS PubMed.
- M. Roca-Ayats, G. García, J. Galante, M. Peña and M. Martínez-Huerta, Int. J. Hydrogen Energy, 2014, 39, 5477–5484 CrossRef CAS.
- J. Wu, S. Mallikarjun Sharada, C. Ho, A. W. Hauser, M. Head-Gordon and A. T. Bell, Appl. Catal., A, 2015, 506, 25–32 CrossRef CAS.
- We note, however, that Ir atoms on bulk PtIr surfaces are assumed to actively participate in fragmentation reactions, see ref. 68. For the sake of simplicity in the direct comparison between various bimetallic structures these optional reaction pathways are not covered in the current study.
- Q. Qi, X. Wang, L. Chen and B. Li, Appl. Surf. Sci., 2013, 284, 784–791 CrossRef CAS.
- The following spin multiplicities are found for the ground states of the tetrahedral bimetallic Pt3X clusters: singlet for Si, Ge and Sn, doublet for In, Ga, Al, Au, Ag and Cu, triplet for Pt and Pd, and quartet for Ir.
- A series of preliminary calculations showed that the first transition states remain rate-determining, even if a partial hydrogen coverage of the catalysts is assumed.
- S. Furuyama, D. M. Golden and S. W. Benson, J. Chem. Thermodyn., 1969, 1, 363–375 CrossRef CAS.
- D. A. Pittam and G. Pilcher, J. Chem. Soc., Faraday Trans. 1, 1972, 68, 2224–2229 RSC.
- L. Nykänen and K. Honkala, J. Phys. Chem. C, 2011, 115, 9578–9586 Search PubMed.
- We note that the inclusion of a dispersion correction to the density functional reduces the barrier heights for continued C–H breaking relative to the energy needed for propene desorption, predicting a reduced selectivity towards the desired product (see ESI†).
- R. Z. Khaliullin, A. T. Bell and M. Head-Gordon, J. Chem. Phys., 2008, 128, 184112 CrossRef PubMed.
- J. N. Bronsted, Chem. Rev., 1928, 5, 231–338 CrossRef CAS.
- M. G. Evans and M. Polanyi, Trans. Faraday Soc., 1938, 34, 11–24 RSC.
- Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knzinger and J. Weitkamp, Wiley-VCH Verlag GmbH, Weinheim, Germany, 1997 Search PubMed.
- F. Abild-Pedersen, J. Greeley, F. Studt, J. Rossmeisl, T. Munter, P. Moses, E. Skúlason, T. Bligaard and J. Nørskov, Phys. Rev. Lett., 2007, 99, 016105 CrossRef CAS PubMed.
- V. Pallassana and M. Neurock, J. Catal., 2000, 191, 301–317 CrossRef CAS.
- J. Nørskov, J. Catal., 2002, 209, 275–278 CrossRef.
- A. Michaelides, Z. P. Liu, C. J. Zhang, A. Alavi, D. A. King and P. Hu, J. Am. Chem. Soc., 2003, 125, 3704–3705 CrossRef CAS PubMed.
- L. C. Grabow, F. Studt, F. Abild-Pedersen, V. Petzold, J. Kleis, T. Bligaard and J. K. Nørskov, Angew. Chem., Int. Ed., 2011, 123, 4697–4701 CrossRef.
- A. Logadottir, T. H. Rod, J. K. Nørskov, B. Hammer, S. Dahl and C. J. H. Jacobsen, J. Catal., 2001, 197, 229–231 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp00360e |
| ‡ Current address: Graz University of Technology, Institute of Experimental Physics, Petersgasse 16, 8010 Graz, Austria. |
| This journal is © the Owner Societies 2016 |