Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A ‘bottom up’, ab initio computational approach to understanding fundamental photophysical processes in nitrogen containing heterocycles, DNA bases and base pairs†
Barbara
Marchetti
a,
Tolga N. V.
Karsili
*ab,
Michael N. R.
Ashfold
*a and
Wolfgang
Domcke
b
aSchool of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS, UK. E-mail: tolga.karsili@bristol.ac.uk; mike.ashfold@bristol.ac.uk; Tel: +44 (0)117 928 8312
bDepartment of Chemistry, Technische Universität München, Lichtenbergstr. 4, 85748 Garching, Germany
First published on 8th March 2016
Abstract
The availability of non-radiative decay mechanisms by which photoexcited molecules can revert to their ground electronic state, without experiencing potentially deleterious chemical transformation, is fundamental to molecular photostability. This Perspective Article combines results of new ab initio electronic structure calculations and prior experimental data in an effort to systematise trends in the non-radiative decay following UV excitation of selected families of heterocyclic molecules. We start with the prototypical uni- and bicyclic molecules phenol and indole, and explore the structural and photophysical consequences of incorporating progressively more nitrogen atoms within the respective ring structures en route to the DNA bases thymine, cytosine, adenine and guanine. For each of the latter, we identify low energy non-radiative decay pathways via conical intersections with the ground state potential energy surface accessed by out-of-plane ring deformations. This is followed by summary descriptions and illustrations of selected rival (electron driven H atom transfer) non-radiative excited state decay processes that demand consideration once the nucleobases are merely components in larger biomolecular systems like nucleosides, and both individual and stacked base-pairs.
1. Introduction
Many recent experimental and theoretical studies have sought to explore the photophysics of simple heteroatom containing aromatic molecules1–7 like phenols8–28 and azoles29–39 following excitation in the ultraviolet (UV) spectral region. Such systems are often termed prototypical, on the basis that a thorough understanding of their response to photoexcitation might well offer a useful guide to the photochemistry and photophysics of more complex and more biologically relevant molecules. Detailed understanding of their deactivation processes following UV excitation has thus long been seen as paramount. The first few excited states of these molecules can be partitioned in several different ways. One distinction is obvious; any given orbital promotion will support both singlet and triplet excited states. Unless stated otherwise, this Perspective focuses solely on the decay of singlet excited state molecules, since intersystem crossing to triplet states is not viewed as a significant decay process for the molecules and the (generally isolated molecule) conditions featured in this Perspective.The nature of the orbital populated by photoexcitation is another key distinguisher. The highest occupied molecular orbital (HOMO) in the ground electronic (S0) state of these molecules ranges between a (bonding) ring-centred π orbital or a (largely non-bonding) n orbital centred on the heteroatom, whereas the orbital populated by photoexcitation may be a (ring-centred, anti-bonding) π* orbital or a σ* orbital localised on the heteroatom – to which we return later. π* ← π transitions typically have the largest absorption cross-sections and dominate the UV absorption spectrum; the diabatic 1ππ* states that result are often termed optically ‘bright’. π* ← n transitions are generally weaker, but much of the recent interest1–5,7 in these molecules has centred on the (hitherto often neglected) excited states formed by σ* ← π (or σ* ← n) promotions which, for brevity, we will henceforth describe simply as 1πσ* excited states. The 1πσ* states generally have much smaller absorption cross-sections and, as a result, are sometimes described as optically ‘dark’.
The energetic ordering of the first excited 1πσ*, 1nπ* and 1ππ* states (i.e. the 11πσ*, 11nπ* and 11ππ* states) is molecule dependent. In the case of azoles like pyrrole1,40 and imidazole,41–43 the 11πσ* state is lowest in energy, whereas in the phenols2 the 11ππ* state is the lowest energy excited state in the Franck–Condon (FC) region. The σ* orbital in these first row heterocycles has significant Rydberg (3s) character in the FC region, but acquires progressively more anti-bonding character upon X–H (X = N, O, etc.) bond extension. As a result, given the relative 11πσ* ← S0 excitation and X–H bond dissociation energies, the potential energy surfaces (PESs) for these 11πσ* states are repulsive with respect to increasing RX–H. Exciting molecules like imidazole at long UV wavelengths populates the 11πσ* excited state directly and results in prompt N–H bond fission. Long wavelength excitation of phenol, in contrast, populates the 11ππ* state which, at the diabatic level, is bound but can predissociate by coupling with the 11πσ* state and subsequently breaking the O–H bond on a much longer timescale.16,19
Bond fission is just one of the several possible decay pathways available to the excited state molecules considered in this Perspective. Almost all of these pathways involve radiationless transfer from the initial state populated by photoexcitation (e.g. between different 1ππ* and/or 1nπ* states, from a 1nπ* or 1ππ* state to a 1πσ* state, or from a 1nπ* or 1ππ* state to the S0 state). As discussed below, the strength of such couplings maximises at molecular geometries where the relevant PESs become near degenerate – so called regions of conical intersection (CI).
The DNA/RNA bases illustrate this progressive increase in complexity nicely. These molecules show strong π* ← π absorptions at wavelengths ∼260 nm,44,45 excitation of which could potentially induce photoreactions and harmful structural changes. Yet the DNA bases and base pairs display well-documented photostability.46–53 This resistance to photodamage is attributable to ultrafast and efficient non-radiative (and non-reactive) relaxation to the S0 state via internal conversion (IC). Femtosecond time-resolved studies of the isolated bases – in the gas phase54–65 and in aqueous solution66–77 – return excitation energy and environment dependent excited state lifetimes but, in all cases, these lifetimes are short; radiationless transfer to the S0 state in the isolated bases typically occurs on a (sub)picosecond time scale. Ab initio calculations attribute such efficient excited state decay to the presence of low energy CIs between the excited (1ππ*) and S0 state PESs along out-of-plane deformation (ring puckering) coordinates.78–107
Here we seek to explain, and to systematise, various of the non-radiative excited state decay processes available to related families of nitrogen containing heterocycles by pooling new results obtained using a range of ab initio electronic structure methods with existing literature data. Without question, the existing literature contains reports of more comprehensive and detailed computational studies of several of the systems covered in this Perspective, and we have sought to cite these at appropriate points in the narrative. The prime focus of the present study is the systematic analysis of the excited state photophysics of families of related molecules investigated in an (as best as possible) even handed and self-consistent manner.
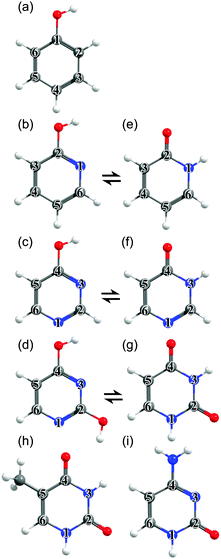
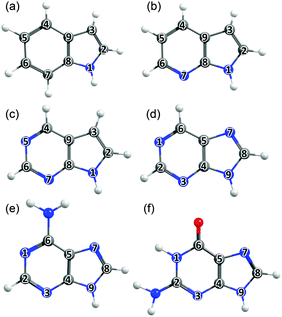
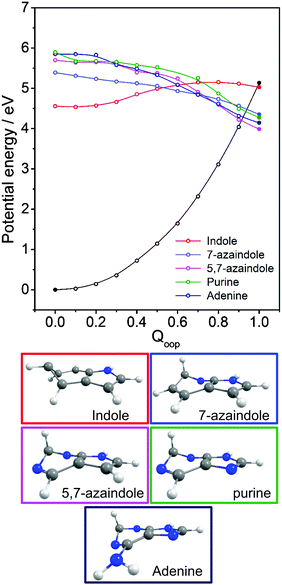
The philosophy of the study is as follows. Uracil (U), thymine (T) and cytosine (C) are pyrimidine derivatives, and hydroxypyridines and hydroxypyrimidines relate to phenol if one or more of the ring C atoms are replaced by N atoms. Adenine (A) and guanine (G) are purine derivatives. Purine itself can be viewed as the result of substituting three of the ring C atoms in indole by N atoms, and indole comprises imidazole fused with a benzene ring. This Perspective thus starts by considering trends in the excited state photophysics of the family of unicyclic molecules shown in Fig. 1, wherein C atoms in phenol are progressively replaced by N atoms, leading through selected pyridines and pyrimidines en route (after appropriate tautomerism and/or derivitization) to U, T and C. This is followed by a similar analysis of the evolving excited state photophysics of the family of bicyclic molecules shown in Fig. 2 as we step from indole through two azaindoles and purine to A and G. Both studies serve to illustrate the progressive lowering of the energies of 1ππ*/S0 CIs in out-of-plane deformation coordinates brought about by incorporating N atoms in the ring. The Perspective concludes by highlighting additional non-radiative decay pathways (most notably excited state H atom transfer processes) that demand consideration as the molecular size and complexity increases further – as exemplified by the ribonucleosides (Fig. 3) and the base-pairs (Fig. 4).
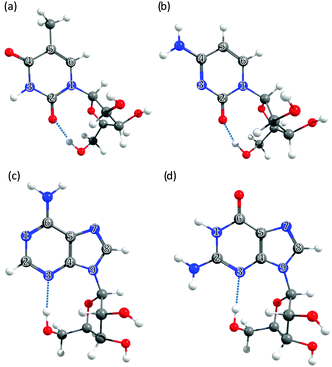 | ||
| Fig. 3 The nucleosides: (a) 5-methyluridine, (b) cytidine, (c) adenosine and (d) guanosine, with the ring atoms of the base numbered for future reference. | ||
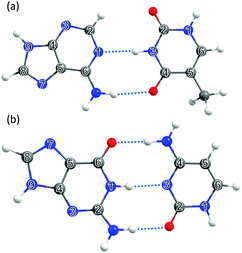 | ||
| Fig. 4 The DNA and RNA base pairs: (a) adenine–thymine (A–T) and (b) guanine–cytosine (G–C), with the ring atoms in the respective bases again numbered for future reference. | ||
2. Theoretical methodology
A brief overview of the ab initio computational methods used in the present calculations is given here. Fuller details can be found in the ESI.†2.1 Ground state tautomerism
Using the Gaussian09 computational package,108 the ground state tautomers were assessed by optimising, where appropriate, the enol and keto forms along with the transition state (TS) connecting the two structures using Møller–Plesset second order perturbation theory (MP2) along with Dunning's augmented correlation consistent basis set of triple-ξ quality (AVTZ).109 The minimum energy path (MEP) for tautomerism was computed at the DFT/CAM-B3LYP/AVTZ level of theory using the intrinsic reaction coordinate (IRC) algorithm embedded within Gaussian 09.2.2 Individual bases
The geometries of the ground state minimum, all conical intersections between the S0 and 1ππ* PESs in the out-of-plane distortion coordinate, Qoop, and (where appropriate) any TSs of interest were optimised using Complete Active Space-Self Consistent Field (CASSCF) methods coupled with the 6-31G(d) basis set using Gaussian09.110 All possible out-of-plane deformation S0/1ππ* CIs were initially optimised. In each case, attention then focussed on the minimum energy CI (MECI) and subsequent linear interpolations in internal coordinates (LIICs) were constructed between the S0 minimum and this MECI. The energies along this LIIC were then calculated using Complete Active Space with second-order Perturbation Theory (CASPT2) with a cc-pVDZ (VDZ) or AVDZ basis set, using the MOLPRO v2010.1 computational package.111 For the specific cases of phenol, 2-hydroxypyridine and 2-pyridone, unrelaxed CASPT2/AVDZ scans were also calculated along the RX–H (X = O or N) coordinate.2.3 Nucleosides and base pairs
The S0 state geometries of the nucleosides were optimised using MP2/6-31G(d) whilst the S0/1ππ* CI geometries were optimised using CASSCF/6-31G(d). MP2/6-31G(d) and CASSCF/6-31G(d) were also used to optimise the geometries of the S0 minimum and the S0/1ππ* CI, respectively, for the A–T base pair. All MP2 and CASSCF calculations were carried out using Gaussian09. In each case, potential energy profiles along the LIIC linking these optimised critical points were constructed at the CASPT2/VDZ level of theory using the MOLPRO v2010.1 computation package. Relaxed potential energy profiles along the electron-driven proton (hydrogen) transfer (EDPT) coordinate of current interest were calculated for these four nucleosides and the A–T and G–C base pairs using ADC(2)/VDZ in Turbomole v6.3112 for both the S0 and S1 (1nπ*(charge transfer (CT))) states. In the nucleosides, the driving coordinate of interest in each case was the terminal ribose sugar O–H bond whereas, in the base pairs, it was the individual base centred O–H or N–H bonds.2.4 Radical-anion pairs
Calculations on the G–C and A–T radical-anion base pairs were performed in order to mimic inter-stack electron transfer. The relaxed ground state potential energy profiles of the (adenine–thymine)− and (guanine–cytosine)− radical-anion base pairs were computed at the TD-DFT/CAM-B3LYP/VDZ level of theory using RN−H as the driving coordinate in Gaussian09.3. Results and discussion
The material in this section is structured as follows: several of the target molecules can exist as both keto- and enol-tautomers, and Section 3.1 reviews how substituting C atoms in the ring by N atoms affects the relative stabilities of these tautomers in the S0 state. The rest of the Perspective focusses on excited states. Sections 3.2 and 3.3 explore how the stabilities and non-radiative decay dynamics of the families of molecules displayed in Fig. 1 and 2 are influenced by C ↔ N atom substitution within the ring. Sections 3.4 and 3.5 then explore some of the additional non-radiative decay pathways (H atom transfer processes) that can arise in the selected nucleosides and base pairs shown in Fig. 3 and 4.3.1 Ground state tautomerism
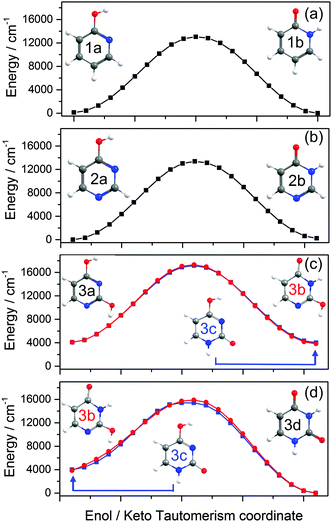
Table 1 compares the ground state energies of 2-hydroxypyridine, 4-hydroxypyrimidine and 2,4-dihydroxypyrimidine with those of the corresponding keto-tautomers calculated at several different levels of theory. The respective stabilities listed in Table 1 are all referenced to the more stable tautomer.| Level of theory | 2-Hydroxypyridine/2-pyridone | 4-Hydroxypyrimidine/4-pyrimidone | 2,4-Dihydroxypyrimidine/uracil | ||
|---|---|---|---|---|---|
| ΔE/cm−1 (1a–1b) | ΔE/cm−1 (2b–2a) | ΔE/cm−1 | |||
| (3d–3a) | (3d–3c) | (3d–3b) | |||
| DFT/CAM-B3LYP/AVDZ | 110 (E) | 240 (K) | 4090 (K) | 4000 (K) | 3830 (K) |
| DFT/CASSCF(10,8)/AVDZ | 100 (E) | 690 (K) | — | — | — |
| MP2/AVTZ | 840 (E) | 260 (E) | 3370 (K) | 3760 (K) | 3470 (K) |
| DF-MP2-F12/AVTZ | 800 (E) | 230 (E) | 3450 (K) | 3780 (K) | 3530 (K) |
| CCSD/AVTZ | 420 (E) | 120 (K) | 3890 (K) | 4050 (K) | 3650 (K) |
| CCSD(F12*)/AVTZ | 410 (E) | 130 (K) | 3970 (K) | 4080 (K) | 3710 (K) |
| CCSD(T)(F12*)/AVTZ | 390 (E) | 100 (K) | 3710 (K) | 3860 (K) | 3550 (K) |
| CASPT2(12,11)/AVTZ | 1130 (E) | 390 (K) | — | — | — |
| Experiment | 270 (E)112 | 167 (K)115,116 | — | — | (K)117 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) barrier to tautomerism in the isolated molecule. The greater relative stability calculated for the enol tautomer is consistent with its larger number of resonance structures over which the π electron density can be delocalised. In solution, however, the relative stabilities of the two tautomers are very sensitive to the solvent polarity; in aqueous solution, for example, the stabilisation from hydrogen bonding ensures that the keto-form is the majority tautomer.114,115
000 cm−1) barrier to tautomerism in the isolated molecule. The greater relative stability calculated for the enol tautomer is consistent with its larger number of resonance structures over which the π electron density can be delocalised. In solution, however, the relative stabilities of the two tautomers are very sensitive to the solvent polarity; in aqueous solution, for example, the stabilisation from hydrogen bonding ensures that the keto-form is the majority tautomer.114,115
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the keto form is able to accept and stabilise additional π density introduced in the 1-position, whereas an OH group in the 4-position (as in the enol form) would tend to be destabilised.
O group in the keto form is able to accept and stabilise additional π density introduced in the 1-position, whereas an OH group in the 4-position (as in the enol form) would tend to be destabilised.
In cytosine, for example, there are fourteen possible tautomer/rotamers, five of which are shown in Fig. 6(a)–(e). The 1H-keto-amino form (Fig. 6(a)) is the preferred tautomer in the Watson–Crick (WC) base pairing. H atom migration from the N1–H group to the neighbouring O atom or to the N3 atom gives rise to three distinct tautomers – two 2-enol-amino structures (of which only the anti-rotamer is shown in Fig. 6(b)) and the 3H-keto-amino tautomer (Fig. 6(c)). The relative stabilities of the different tautomeric forms are again sensitive to the chemical environment. Microwave, resonance enhanced multiphoton ionisation (REMPI) and core level photoemission measurements reveal both the 1H-keto-amino and 2-enol-amino tautomers in jet-cooled gas phase samples.119–122 Low temperature matrix isolation studies (in the IR and the UV) also identify both of these tautomers and conclude that the 2-enol-amino tautomer is more abundant,123,124 whereas the 1H-keto-amino form is most stable in aqueous solution.125 The available theoretical predictions are broadly supportive of these conclusions.126–128 Under isolated molecule conditions, the 1H-keto-amino and 2-enol-amino tautomers are calculated to lie lowest in energy (with the former ∼500 cm−1 more stable than the latter), with the two imino (Fig. 6(d) and (e)) and the 3H-keto-amino (Fig. 6(c)) tautomers consistently higher in energy. Micro-solvation with water molecules, under isolated gas-phase conditions,127 is predicted to favour the keto-amino tautomers, particular the 1H-keto-amino structure (calculated as being ∼2040 cm−1 more stable than the 3H-equivalent) and to destabilise the enol and imino tautomers.
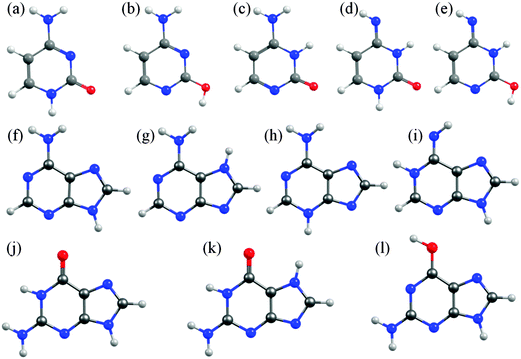 | ||
| Fig. 6 Selected low energy tautomeric forms for cytosine (structures (a)–(e)), adenine (structures (f)–(i)) and guanine (structures (j)–(l)). | ||
In A, the H atom at the N9 position can migrate to any of the other nitrogen atoms within the ring, giving a total of fourteen possible tautomers/rotamers.129 Experimental studies have identified the presence of the 9H-amino (Fig. 6(f)) and, in some cases, the 7H-amino (Fig. 6(g)) tautomers in the gas phase,130–133 in matrix isolation conditions134 and in polar solvent environments (e.g. water).135–138 All of these studies concur that the canonical 9H-amino form is the dominant tautomer in all environments (as it is in DNA itself), though the 7H-amino variant gains some relative stabilisation in polar solvents by virtue of its larger dipole moment. These experimental conclusions have been reinforced by numerous ab initio studies, though most predict a significant (∼2400–3000 cm−1) energy difference between the 9H- and 7H-amino tautomers.130,139,140 The 3H-amino- and the various imino forms (e.g. the 1H,9H-imino structure shown in Fig. 6(i)) are all calculated to be considerably higher in energy.
G is functionalized with both amino and keto groups, and has the greatest number of possible tautomers.141 An early IR matrix isolation study showed G present as a mixture of enol and keto tautomers,142 and more recent studies have identified three different tautomers (one enol and two keto forms) in a supersonic jet.143–146 Guided by complementary ab initio calculations, these were assigned to the 1H,7H-keto-amino (Fig. 6(k)), the 1H,9H-keto-amino (Fig. 6(j)) and the 9H-enol-amino (Fig. 6(l)) tautomers, with the latter likely present as two different rotamers. Theory140,145–148 also predicts that the 1H,7H-keto-amino structure is the most stable as an isolated molecule, followed by the 1H,9H-keto-amino tautomer (with reported energy separations in the range 20–250 cm−1) and then the 9H-enol-amino tautomer (some 270–620 cm−1 above the global minimum structure). All of the other tautomers not shown in Fig. 6 are predicted to lie at least 1000 cm−1 above the global minimum. Once again, however, this energetic ordering is disturbed by solvating with water,146 under which circumstances the tautomeric equilibria are predicted to shift in favour of the 3H,7H-keto-amino (now the most stable), 7H,9H-keto-amino and 3H,9H-keto-amino tautomers.
The primary aim of this Perspective is to seek trends and differences in the excited state photophysics exhibited by these molecules. Thus we choose to focus attention on the tautomeric forms adopted by the various nucleobases in the common WC or in the Hoogsteen base pairing schemes (i.e. 1H-cytosine, 9H-adenine and 1H,9H-guanine, as depicted in Fig. 1(i), 2(e) and (f)).
3.2 Unicyclic ring systems and the pyrimidine bases
| Moleculeref. | ΔE(S1–S0) | ΔE(S2–S0) | ΔE(S3–S0) | ΔE(TS–S0) | ΔE(CI–S0) | |||
|---|---|---|---|---|---|---|---|---|
| VEE | AEE | Exp. | VEE | Exp. | VEE | |||
| Enol | ||||||||
| Phenol9 | 4.57 (ππ*) | 4.34 | 4.51 (A) | 5.40 (πσ*) | — | — | 4.89 | 4.63 |
| 2-Hydroxy-pyridine154,155,157 | 4.70 (ππ*) | 4.34 | 4.48 (A) | 6.17 (nπ*) | 6.79 (nσ*) | 4.71 | 4.32 | |
| 4-Hydroxy-pyrimidine | 4.94 (nπ*) | 4.64 | — | 5.18 (ππ*) | — | 5.28 (nπ*) | 4.55 | 3.81 |
| 2,4-Dihydroxy-pyrimidine | 4.90 (nπ*) | 4.52 | — | 5.02 (ππ*) | — | 6.43 (πσ*) | — | 3.80 |
| Keto | ||||||||
| 2-Pyridone156,195–197 | 4.34 (ππ*) | 3.57 | 3.70 (A) | 4.81 (nπ*) | — | 5.15 (nσ*) | — | 3.70 |
| 4-Pyrimidone | 4.45 (ππ*) | — | 3.85 (A) | 4.92 (nπ*) | — | 5.58 (nπ*) | — | 3.12 |
| Uracil198 | 4.80 (nπ*) | 4.17 | — | 5.69 (ππ*) | 5.08 (V) | 5.89 (πσ*) | — | 3.92 |
| Thymine199 | 4.85 (nπ*) | 4.17 | — | 5.50 (ππ*) | 4.80 (V) | 5.64 (πσ*) | — | 4.09 |
| Cytosine60,119 | 4.58 (ππ*) | — | — | 4.88 (nπ*) | 3.95 (A) | 5.34 (nπ*) | — | 3.46 |
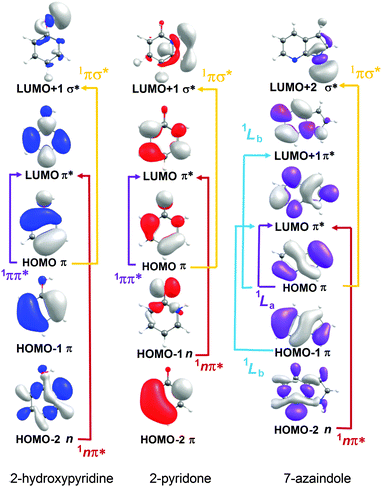 | ||
| Fig. 7 Dominant orbital promotions involved in forming the first few singlet excited states of 2-hydroxypyridine, 2-pyridone and 7-azaindole. | ||
As discussed in Section 3.1, the molecules formed by introducing one (or more) N atoms into the ring can exist as enol and keto tautomers. The present calculations show the S1 and S2 states of 2-hydroxypyridine, 2-pyridone, 4-pyrimidone to have 1ππ* and 1nπ* character, respectively, in the FC region. However, this energetic ordering is reversed in the cases of 4-hydroxypyrimidine, 2,4-dihydroxypyrimidine, U and T. Fig. 7 illustrates the (expected) better spatial overlap between the orbitals involved in the π* ← π excitations cf. the π* ← n excitations.
Several trends are evident in the data collected in Table 2. Amongst the enols, the 1ππ*-S0 term values are similar in phenol and 2-hydroxypyridine, and larger in 4-hydroxypyrimidine and 2,4-dihydroxypyrimidine. The similar term values in phenol and 2-hydroxypyridine can be understood if the weak intramolecular H-bond between the N atom and the OH at the C2 position in the latter provides a similar stabilisation to both the π and π* orbitals. OH group(s) are π-donors. This additional electron density is preferentially localised at positions ortho- and para- to the ring-atom bearing the OH group, and can be accommodated more readily by a more electronegative N atom (cf. a C atom). The increased 1ππ*-S0 term values in 4-hydroxypyrimidine and 2,4-dihydroxypyrimidine (wherein the 1ππ* state is raised above the 11nπ* state) are thus attributed to preferential stabilisation of the π (and destabilisation of the π*) orbital by the additional N atom. Table 2 also shows that the S1 states of the keto tautomers (2-pyridone and 4-pyrimidone) are formed by the same orbital promotions as the corresponding enols, and that their 1ππ*-S0 term values are consistently smaller, despite the gradual stabilisation of the S0 state of the keto form upon N atom inclusion (recall Table 1). This can be understood by considering the differing π donating capabilities of NH and OH groups, which will be amplified by the fact that the OH group is pendant to the ring. The in-ring N atom in the NH group is much more effective at stabilizing the electron deficiency introduced by π* ← π excitation (and thus the 1ππ*-S0 energy gap) in the keto tautomers.
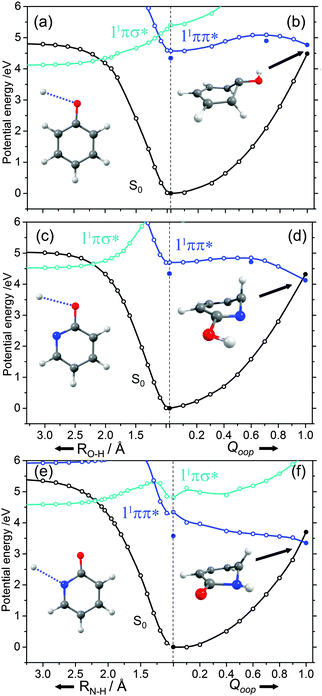
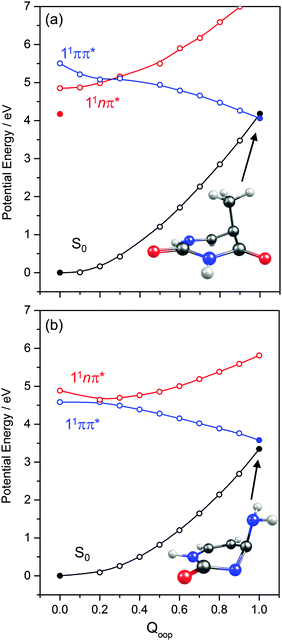
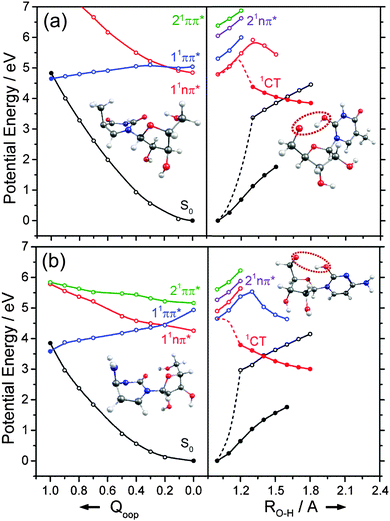
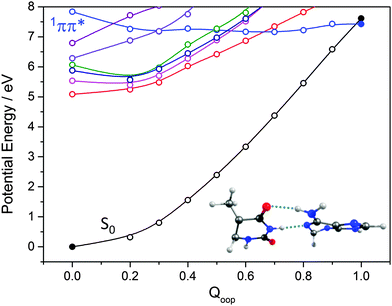
The ns lifetime of the 11ππ* state of phenol implies the absence of any efficient rival non-radiative decay processes. One such non-radiative decay process identified in similar photoexcited molecules is internal conversion to the ground state facilitated by a CI between the 11ππ* and S0 states that can be accessed by motion along an out-of-plane ring deformation coordinate, Qoop (via a so-called prefulvenic geometry).14,149–152Fig. 8(b) shows the calculated geometry of this 11ππ*/S0 MECI in phenol – with C1 very obviously out of the plane. As in later figures of this type, the open points show the ground (and excited) state energies calculated at a sequence of points along the LIIC connecting the optimised S0 state geometry to that of the 11ππ*/S0 MECI. The small number of filled points show the energies of the optimised critical structures (notably the minimum of the 11ππ* state and the energy of any TS separating this minimum excited state geometry from the MECI) calculated at the CASPT2/AVDZ level of theory. These optimised energies are also listed in Table 2. In the specific case of phenol, the key finding from these calculations is the presence of a substantial (∼0.5 eV) energy barrier in the MEP towards this prefulvenic MECI, that would be sufficient to render this pathway closed following excitation at energies not far above the origin of the 11ππ* state.
Fig. 8(c) and (d) show the corresponding PECs along RO–H and the prefulvenic Qoop coordinate for 2-hydroxypyridine. The most obvious feature of the former is the predicted elevation of the 11πσ* potential (or, more precisely, stabilisation of the S0 state by the intramolecular H-bonding interaction between the OH group and the in-plane orbital on the adjacent ring N atom), which manifests itself as an increased barrier to tunnelling under the 11ππ*/11πσ* CI (cf. phenol). Indeed, if the potentials along the LIIC displayed in Fig. 8(c) are anything like quantitative, this barrier would preclude O–H bond fission as a possible decay process following excitation near the minimum of the 11ππ* state of 2-hydroxypyridine. As Fig. 8(d) shows, the predicted MECI along Qoop lies at an energy below that of the 11ππ* minimum but, as in phenol, motion towards this prefulvenic 11ππ*/S0 CI is impeded by an energy barrier (the calculated energy of the optimised TS is ∼0.37 eV above that of the 11ππ* state minimum (see Table 2)). Once again, therefore, theory would predict a certain photophysical stability to the 11ππ* state of 2-hydroxypyridine, consistent with previous theoretical studies153,154 and experimental observations of well-resolved vibronic structure in the jet-cooled REMPI spectrum of this tautomer.155,156 We also note that geminate recombination at the 11ππ*/S0 CI could lead to branching into the S0 state of the keto tautomer via a photoinduced dissociation-association reaction. Though entropically unfavourable under isolated gas-phase conditions, such a process would certainly merit consideration if the chromophore was suspended in a rare-gas matrix or in bulk solution.
Analogous PECs for 2-pyridone are presented in Fig. 8(e) and (f). The profiles along the X–H stretch coordinate (RN–H in this case) show qualitative similarities with those for the 11ππ* and 11πσ* states of phenol (Fig. 8(a)) but the optimised energy of the 11ππ* state of 2-pyridone is well below the N–H dissociation limit, thereby excluding N–H bond fission as a possible decay channel following excitation at energies around the 11ππ*-S0 origin. The predicted MECI in the prefulvenic Qoop coordinate is below the 11ππ* ← S0 vertical excitation energy, however (even after geometry optimisation, see Table 2), and the calculated PEC along the LIIC to the 11ππ*/S0 CI is essentially barrierless. Such predictions also appear to be consistent with the available experimental data: the (split) origin band of the 11ππ*-S0 transition of 2-pyridone shows resolved rovibronic structure and the dispersed emission spectrum following excitation to the origin level(s) reveals population of an extensive range of ground state vibrational levels.157 The latter observation implies that the 11ππ* and S0 states of 2-pyridone have different equilibrium geometries (as is also implied by the difference in the VEE and AEE values reported in Table 2). The reported two colour 1 + 1′ REMPI spectrum via the 11ππ* state, in contrast, is dominated by the origin band(s) – a finding that can most readily be explained by invoking a sharp drop in excited state lifetime upon tuning to higher energies.154,155,158
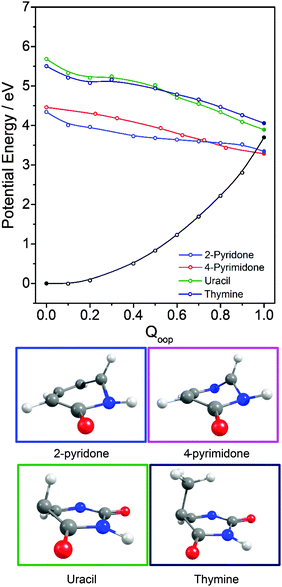
PECs along Qoop for the ground and first three excited singlet states of (a) thymine and (b) cytosine are shown in Fig. 9. Again, these energies were calculated at the CASPT2 level for a sequence of geometries along a LIIC linking the CASSCF optimised S0 minimum and the S0/11ππ* MECI. These values are also reported in Table 2. The similarities between these PECs for T and C, and those for 2-pyridone (Fig. 8(f)), are obvious, notwithstanding the reversal in the energetic ordering of the 11nπ and 11ππ* states. In both T and C, the calculated 11ππ* PEC leading to the 11ππ*/S0 MECI in the Qoop coordinate is barrierless. Indeed, the predicted energy of the 11ππ*/S0 MECI is even below that of the 11nπ origin – consistent with previous observations of ultrafast excited state decay following UV excitation of both T and C.54,55,57,59
Notwithstanding the rich literature addressing the excited state photophysics of the DNA/RNA bases, there have been few systematic studies of the electronic and geometric reasons why such out-of-plane ring deformations are effective in promoting ultrafast excited state decay – and thus photostability – in pyrimidine derivatives like T and C, but not in simpler analogues like phenol. Yet recognising the factors that underpin this photostability could be important in guiding the development of more efficient photoprotective molecules for use in, e.g., sunscreens or artificial intraocular lenses. Thus we now summarise, and seek to rationalise, how the introduction of N atoms into the phenol ring and the addition of further OH and/or NH2 functionalities influences the topography of the excited state PES(s) along Qoopen route to the MECI with prefulvenic geometry.
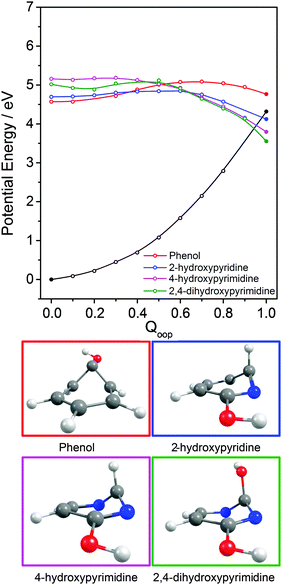
Inspecting these data, we see that the VEEs of the respective 11ππ* states increase across the enol series phenol → 2,4-dihydroxypyrimidine, while the energies of the 11ππ*/S0 MECIs decrease. As a consequence, the barrier to accessing the 11ππ*/S0 MECI decreases across the series and the gradient along Qoop becomes progressively steeper with increasing N incorporation and ring functionalization. The VEEs of the 11ππ* states of the keto tautomers also increase across the series 2-pyridone → U and T.159 The energies of the 11ππ*/S0 MECIs increase in much the same order, but are always below the corresponding VEEs. The 11ππ* PECs leading to these MECIs have similar gradients in all four species, and none shows any obvious barrier. This difference in topography (cf. the enol tautomers) can plausibly be traced to differences in the extent of the π-conjugation in the enol and keto families. The sp2 hybridisation of the N atom in 2-hydroxypyridine, for example, would be expected to be stronger than that in the keto analogue (2-pyridone), and thus more resistant to initial out of plane deformation – consistent with the barrier predicted in the PEC along Qoop in the former.
Various trends revealed in these data are now considered in turn. The ground state of each of these systems is aromatic, the equilibrium geometry of the ring atoms is planar and the potential energy increases with out-of-plane ring deformation. Introducing one or more heteroatoms into the ring will weaken the rigidity, whereas adding OH groups (as in the enols) and, particularly, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (as in the keto tautomers) tends to reinforce the preference for planarity by extending the π-conjugation. π* ← π excitation destroys the aromaticity, induces some expansion of the ring, and lowers the resistance to out-of-plane ring deformation – to the extent that, in all bar phenol, the 11ππ* state potential is either essentially flat, or unstable, with respect to out-of-plane deformation. Under such circumstances, a CI with the S0 PES upon distorting along Qoop is inevitable. As Fig. 10 and Table 2 show, the energy of the 11ππ*/S0 MECI in Qoop decreases upon introducing one or more N atoms into the ring. In all cases other than phenol, the 11ππ*/S0 MECI in Qoop is calculated to lie below the VEE of the 11ππ* (and the 11nπ*) excited states. These MECIs also lie below the calculated O–H and N–H bond dissociation energies in 2-hydroxypyridine and 2-pyridone. Further, recalling Fig. 8(c) and (e), the latter excited state bond fissions are predicted to be hampered by a potential barrier (the lower part of the 11ππ*/11πσ* CI in RX–H (X = N, O)). Thus we conclude that, at least relative to X–H bond fission, IC to the S0 state mediated by out-of-plane distortion is likely to be the dominant decay process following long wavelength excitation of the N-containing heterocycles in Table 2. However, we also recognise that the excited state decay rate will be sensitive not just to the detailed topography of the excited state PES connecting the FC region to the 11ππ*/S0 MECI in Qoop (which may be influenced by substitution as, for example, for the cases of 5-hydroxy- and 5-aminouracil160), but also to the excitation energy.
O groups (as in the keto tautomers) tends to reinforce the preference for planarity by extending the π-conjugation. π* ← π excitation destroys the aromaticity, induces some expansion of the ring, and lowers the resistance to out-of-plane ring deformation – to the extent that, in all bar phenol, the 11ππ* state potential is either essentially flat, or unstable, with respect to out-of-plane deformation. Under such circumstances, a CI with the S0 PES upon distorting along Qoop is inevitable. As Fig. 10 and Table 2 show, the energy of the 11ππ*/S0 MECI in Qoop decreases upon introducing one or more N atoms into the ring. In all cases other than phenol, the 11ππ*/S0 MECI in Qoop is calculated to lie below the VEE of the 11ππ* (and the 11nπ*) excited states. These MECIs also lie below the calculated O–H and N–H bond dissociation energies in 2-hydroxypyridine and 2-pyridone. Further, recalling Fig. 8(c) and (e), the latter excited state bond fissions are predicted to be hampered by a potential barrier (the lower part of the 11ππ*/11πσ* CI in RX–H (X = N, O)). Thus we conclude that, at least relative to X–H bond fission, IC to the S0 state mediated by out-of-plane distortion is likely to be the dominant decay process following long wavelength excitation of the N-containing heterocycles in Table 2. However, we also recognise that the excited state decay rate will be sensitive not just to the detailed topography of the excited state PES connecting the FC region to the 11ππ*/S0 MECI in Qoop (which may be influenced by substitution as, for example, for the cases of 5-hydroxy- and 5-aminouracil160), but also to the excitation energy.
The detailed form of the out-of-plane displacement leading to the 11ππ*/S0 MECI is also molecule dependent. As noted above, π* ← π excitation results in some ‘loosening’ of the ring structure and localisation of the π-bonding, with one or more ring atoms tending towards a pyramidal bonding arrangement. Among the enols, the atom exhibiting the greatest out-of-plane distortion (defined relative to the best-fit plane through the six ring atoms at the calculated geometry of the 11ππ*/S0 MECI) varies between C1 in phenol, C6 in 2-hydroxypyridine and C2 in 4-hydroxypyrimidine and 2,4-dihydroxypyrimidine – as shown in Fig. 10 and Fig. S1–S4 (ESI†). The 11ππ*/S0 MECI identified in phenol has a classic prefulvenic structure, with C1 (i.e. the C atom supporting the OH group) showing the greatest out-of-plane distortion, while the finding that the C2 atom in both pyrimidines shows the greatest out-of-plane distortion reflects its location between the two N atoms, which more readily adopt a local sp3 (pyramidal) coordination. The carbonyl group(s) in the keto tautomers encourage local planarity; relative to 2,4-dihydroxypyrimidine, C2 in U (and T) is less distorted out of the plane, with strain relief provided, instead, by increased puckering at C5 and C6.
The VEEs of the 11nπ* states of 4-hydroxypyrimidine, 2,4-dihydroxypyrimidine, U and T are all calculated to be below those of the corresponding 11ππ* states (see Table 2) and, as Fig. 9 showed (for the specific cases of T), the respective PESs show a CI in the Qoop coordinate. As suggested previously,78–81,86,87,91,94,103 IC to the 11nπ* state could thus constitute another possible non-radiative decay pathway following excitation to the ‘bright’ 11ππ* state in these molecules. Conversely, one might also envisage instances where IC to the 11ππ* state (and subsequent coupling to S0via the 11ππ*/S0 MECI) could provide a non-radiative population loss route following excitation to higher vibrational levels within the 11nπ* manifold.
3.3 Bicyclic ring systems and the purine bases
Calculated VEEs for the first three excited singlet states of each molecule shown in Fig. 2(a)–(f) are listed in Table 3, along with the dominant character of the various excitations (in parenthesis). The excited states in each case comprise two 1ππ* states, traditionally labelled 1La and 1Lb, and a 1nπ* state. Apart from in the case of indole, the lowest lying 1πσ* excited state has not been included in the present calculations, but H atom photofragment translational spectroscopy measurements following near UV photolysis of gas phase indole33 and adenine161 molecules suggest that the threshold energy for N–H bond fission following radiationless transfer to the 11πσ* state in these molecules is ∼4.71 eV and ∼5.33 eV, respectively.| Moleculeref. | ΔE(S1–S0) | ΔE(S2–S0) | ΔE(S3–S0) | ΔE(TS–S0) | ΔE(CI–S0) | |||
|---|---|---|---|---|---|---|---|---|
| VEE | AEE | Exp. | VEE | Exp. | VEE | |||
| a The relaxed S2/11nπ* state energy is 4.35 eV. | ||||||||
| Indole33,200 | 4.55 (ππ*) | 4.44 | 4.37 (A) | 5.07 (ππ*) | 4.54 (A) | 6.82 (πσ*) | 5.17 | 5.06 |
| 7-Azaindole201–204 | 4.61 (ππ*) | 4.33 | 4.29 (A) | 5.01 (ππ*) | 4.32 (A) | 5.20 (nπ*) | — | 4.28 |
| 5,7-Azaindole | 4.50 (nπ*) | — | 4.97 (ππ*) | — | 5.11 (ππ*) | — | 3.82 | |
| Purine205 | 4.70 (nπ*) | 4.59 | 3.88 (A) | 4.99 (ππ*) | — | 5.68 (ππ*) | — | 4.01 |
| Adenine160–206 | 4.86 (nπ*) | 4.40 (A) | 5.13 (ππ*) | 4.48 (A) | 5.28 (ππ*) | — | 3.90 | |
| Guanine207 | 5.25 (ππ*) | — | 4.13 (A) | 5.55 (nπ*)a | — | 6.53 (nπ*) | — | 3.47 |
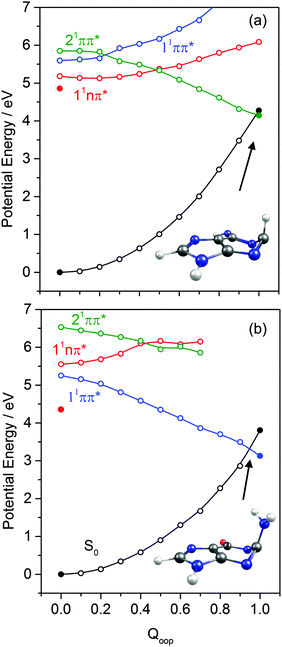
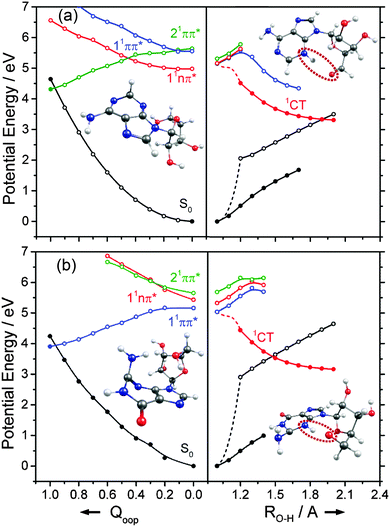
The dominant orbital promotions involved in forming these excited states in 7-azaindole were shown in Fig. 7. The 1La and 1Lb states differ in the detail of the participating π and π* orbitals. The 1La ← S0 transitions are generally pictured as π*(LUMO) ← π(HOMO) excitations and have the larger absorption cross-section.162 As in the pyrimidine systems, however, the energetic ordering of the 1ππ* (1La and 1Lb) and 1nπ* states is sensitive to N substitution (as Table 3 shows, the 11nπ* state is the S1 state in 5,7-azaindole, purine and A) and can be modified by solvation.163 The relative excited state energies are also sensitive functions of Qoop, as shown for the cases of A and G in Fig. 12.
Viewed in terms of these diabatic PECs in Qoop, we find that the higher energy 21ππ*(1La) PEC correlates with the 1ππ*/S0 MECI in 5,7-azaindole, purine, A and G, whereas in indole and 7-azaindole it is the 11ππ*(1Lb) PEC that correlates with this MECI. Fig. 13 compares calculated PECs along the LIIC linking the relevant (vertical) 1ππ* excited state and the 1ππ*/S0 MECI for indole, 7-azaindole, 5,7-azaindole, purine and A. Each is referenced to a common zero of energy (the respective S0 minimum) but again, to avoid congestion, only the S0 PEC for indole is shown. More detailed depictions of the ground and excited state PECs for these molecules, and cross-sectional views showing the out-of-plane displacements of the ring atoms at each of the 1ππ*/S0 MECIs are provided in Fig. S10–S15 in the ESI.† The optimised energies of the various 1ππ*/S0 MECIs (ΔE(CI–S0)) and of any TS identified in this PEC along Qoop (ΔE(TS–S0)) are included in Table 3.
Many of the trends evident in Fig. 13 are reminiscent of those discussed previously for the unicyclic systems. The 1ππ*/S0 MECI in indole is calculated to lie above the AEE of the 11ππ* state. Motion along Qoop is thus unlikely to promote IC following photoexcitation at the 11ππ*(1Lb) ← S0 origin – consistent with the reported (∼17 ns) excited state lifetime.164,165 As Fig. 13 shows, however, the introduction of even one N atom into the six-membered ring raises the VEE of the 1ππ* state and lowers the energy of the 1ππ*/S0 MECI sufficiently that the PEC linking the FC region to the 1ππ*/S0 MECI in Qoop becomes essentially barrierless. Notwithstanding that in most cases it is the diabatic 21ππ* PEC that links to this MECI, the available literature166 supports the expectation that molecules excited to (vibrationally excited levels of) the 11ππ* and 11nπ* states will find efficient radiationless coupling pathways to this (and related) CIs with the S0 PES.
The out-of-plane displacements required to access the various 1ππ*/S0 MECIs also have obvious parallels with those discussed in Section 3.2. Again, we choose to define these displacements with respect to the plane of best-fit through the (nine in this case) ring atoms. As Fig. 13 and Fig. S10–S15 (ESI†) show, the distortions are largely concentrated in the 6-membered ring. The atom showing the largest out-of-plane displacement at the 1ππ*/S0 MECI is again molecule dependent, but the largest distortions are always remote from the bond that fuses the two ring systems. C5 is most displaced in indole, but this switches to C6 in 7-azaindole (reflecting the energetic preference for sp3 hybridising the neighbouring N7 atom). The 1ππ*/S0 MECI in 5,7-azaindole is characterised by an obvious puckering around N5 and C6. Introducing an additional N atom in the 5-membered ring (as in purine, A and G) leads to a switch in the numbering convention used when describing the imidazole containing bicyclic systems – recall Fig. 2. It also allows greater relaxation of the bond common to the two rings (the C4–C5 bond in these molecules), with the result that C2 located between the N1 and N3 atoms is predicted to show the largest out-of-plane displacement at the 1ππ*/S0 MECI – as in the unicyclic analogues 4-hydroxypyrimidine, 2,4-dihydroxypyrimidine and 4-pyrimidone.
3.4 The ribonucleosides and deoxyribonucleosides
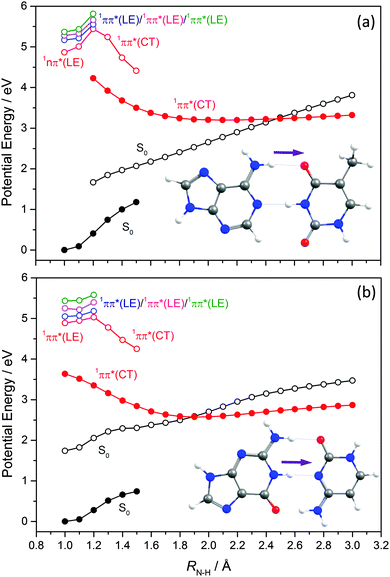
We now turn to the individual RNA base nucleosides: 5-methyluridine (uridine, thymidine), cytidine, adenosine and guanosine. The present study is limited to the oxygenated nucleosides with a ribose sugar attached to the N terminus of each base, but replacing one OH group on the ribose sugar by an H atom (as in the deoxyribonucleosides) is not expected to have any substantial effect on the excited state photophysics described in this section. The CASPT2 calculated PECs along Qoop displayed in Fig. 14 and 15 exhibit obvious similarities with those of the precursor base. For comparative purposes, we have also scanned the Qoop coordinate of adenosine using the ADC(2)/cc-pVDZ level of theory. The resulting PECs, shown in Fig. S34 of the ESI,† are reassuringly similar to those calculated at the CASPT2/cc-pVDZ level.The present calculations suggest that the S1 states of adenosine, 5-methyluridine and cytidine have predominant 1nπ* character in the FC region, whereas that of guanosine is largely of 1ππ* character. In each case we identify a 1ππ*/S0 MECI with prefulvenic geometry lying at an energy below the calculated S1 state minimum. The calculated 1ππ* PEC from the FC region to this MECI is in all cases barrierless. Such findings reinforce the view that the nuclear deformations required to sample this MECI are base-centred and suggest that the oxygenated ribose sugar is largely a spectator in this particular radiationless pathway. More detailed inspection reveals that the energies of the various excited states and the prefulvenic 1ππ*/S0 MECIs in the nucleosides are slightly destabilised relative to the corresponding quantities in the isolated base. These relative increases in excitation energy can be traced to the stabilising effects of hydrogen bonding between an O–H group of the ribose sugar and the base centred N or O atom in the ground state nucleoside.
Such H-bonding interactions are potentially important in the present context, as they can promote rival radiationless decay pathways in the DNA base residues, wherein the photoexcited state of interest develops charge-transfer (CT) character and undergoes intramolecular electron-driven proton (hydrogen) transfer (EDPT) from the terminal O–H group of the ribose to an N or O atom in the aromatic ring. In contrast to the PECs along Qoop – which were calculated at the CASPT2 level to afford fairest comparison with the corresponding PECs for the smaller systems reported in Sections 3.1–3.3 – the PECs along the EDPT coordinate shown in the right hand panels of Fig. 14 and 15 were calculated at the ADC2 level. This is a pragmatic choice. The electronic states that promote EDPT involve orbital promotions that are sensitive to small changes in nuclear geometry. As such, a poorly chosen active space in CASSCF/CASPT2 can lead to spurious results along the charge-transfer coordinate, usually associated with important orbitals entering/leaving the active space. One way to circumvent this is to increase the active space substantially, but this leads to a large increase in computational expense. Given the available computational resources and (relatively) large size of the nucleoside molecules, the increased computational demands would have precluded detailed mechanistic study along the EDPT path with CASPT2 − especially in mapping the relaxed excited state paths.
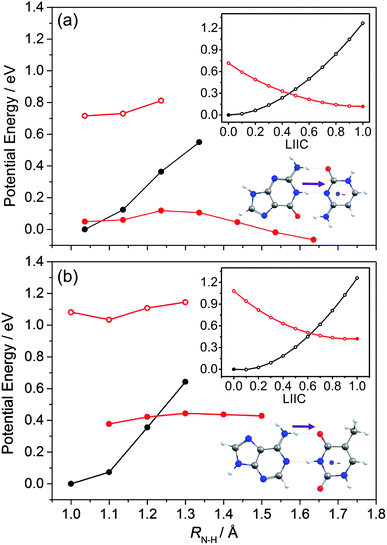
The PECs along the EDPT coordinate were derived as follows: the S0 state was first scanned using the sugar O–H bond as the driving coordinate (henceforth identified simply as RO–H). The filled black points in each panel depict the ‘relaxed’ S0 state energies calculated by fixing RO–H at selected values and allowing all other internal degrees of freedom to relax to their minimum energy configuration. The open coloured points show the various excited state energies calculated at the corresponding S0 state geometries. A relaxed scan was then constructed for the 1CT state (filled red points), and the corresponding S0 state energies calculated at the relaxed S1 geometries (shown by the open black symbols). The dashed black and red curves show the potential energy profiles for, respectively, the ground and first (adiabatic) excited states along the LIIC connecting the S0 minimum geometry and the relaxed 1CT structure at the shortest RO–H distance. In all cases, the energy along the S0 optimised reaction path rises while that of the 1CT state declines with increasing RO–H, with the result that the two states become degenerate at extended O–H bond lengths. The resulting 1CT/S0 CI is consistently calculated to lie at energies below the relevant S1 minimum, and could thus provide an alternative radiationless route to the S0 state following UV excitation (i.e. could provide another route to photostability).
Radiationless transfer via such a low energy 1CT/S0 CI was recently suggested as a possible explanation for experimental reports167 that the excited state lifetime of adenosine is noticeably shorter than that of bare adenine. The calculated PECs along the O–H⋯N3 EDPT coordinate of adenosine (Fig. 15(a)) agree well with the previous calculations by Tuna et al.168 These show that, following vertical excitation to the ‘bright’ 1Lb state, there are barrierless pathways to lower energy CIs with the S0 state in both the Qoop and EDPT coordinates. The 1CT/S0 CI is calculated to lie at lower energy and, given that it is reached by intramolecular transfer of a light H atom, could very well constitute a competitive non-radiative decay pathway following UV excitation. We note the obvious analogy between such a 1CT/S0 CI in an EDPT coordinate and the previously discussed 11πσ*/S0 CI in the RO–H stretch coordinate in phenols. The former will generally be stabilised (and thus potentially active at lower excitation energies), however, given the presence of an acceptor O (or N) site on the aromatic ring.
The other nucleosides also show a low energy 1CT/S0 CI in the EDPT coordinate. The calculated PECs leading to the 1CT/S0 CI in guanosine, for example (right hand panel in Fig. 15(b)), have very similar profiles to those for adenosine. So, too, do the respective PECs linking the FC region to the 1ππ*/S0 MECI along Qoop (left hand panel in Fig. 15(b)). The calculated PECs in the Qoop coordinate show obvious similarities with those of the corresponding base (guanine, Fig. 12(b)). Again, the excited state and CI energies are somewhat elevated in the nucleoside – reflecting the stabilisation of the ground state by intramolecular H-bonding. As in adenosine, therefore, theory suggests that IC via the 1CT/S0 CI could be a competitive photoprotection pathway following UV photoexcitation of guanosine.
Analogous 1CT/S0 CIs have been identified in the respective EDPT coordinates for 5-methyluridine and cytidine also (Fig. 14(a) and (b)). In the specific case of 5-methyluridine, however, the present calculations return an energy barrier in the PEC linking the FC region and the 1CT/S0 CI, which is also calculated to lie at relatively higher energy than in the other nucleobases. For both 5-methyluridine and cytidine, the PEC along Qoop leading to the 1ππ*/S0 MECI with a prefulvenic geometry is calculated to be barrierless. Based on the present limited set of calculations, it is thus tempting to suggest that: (i) EDPT will play a greater relative role in coupling photoexcited cytidine, adenosine and guanosine molecules back to their respective S0 states; and (ii) the non-radiative decay of 5-methyluridine (uridine), and the excited state lifetimes of this nucleoside, should be largely determined by coupling at the 1ππ*/S0 CI in Qoop and thus similar to that of T (U). Zgierski and Alavi reached broadly similar conclusions in prior computational studies of cytidine and bare cytosine.169 However, recent time-resolved pump–probe studies of gas-phase adenosine, cytidine and 5-methyluridine return excited state lifetimes that are, in all cases, shorter than those of the corresponding isolated nucleobase.170 This, the authors propose, reflects the additional excited state decay via EDPT enabled by the sugar backbone, which is not present in the isolated nucleobases.169 Clearly, there is a case for further, higher level theoretical studies of the EDPT decay path in all of the nucleosides.
3.5 The DNA and RNA base pairs
EDPT has also been identified as an efficient deactivation pathway following photoexcitation of WC DNA/RNA base pairs. In the case of the WC A–T base pair,171,172ab initio studies identified a 1ππ* CT state arising via electronic promotion from an A-centred π orbital to a T-centred π* orbital in addition to a number of locally excited (LE) 1nπ* and 1ππ* states (i.e. states formed by electron promotion localised on just A or T). Excitations of the former type lead to charge separation, which is countered by excited state proton transfer.Key PECs along this EDPT coordinate (i.e. extending one of the amine-centred N–H bonds attached to C6 in A towards the O attached to C4 in T) are shown in Fig. 16(a). Again, the filled black points show the ‘relaxed’ S0 energies calculated by progressively extending this N–H driving coordinate and, at each RN–H value, allowing all other internal degrees of freedom to relax to the minimum energy configuration. The open coloured points show the various excited state energies calculated at the S0 geometries returned in this way. The filled red points define the ‘relaxed’ PEC for the 1CT state, and the open black symbols show the corresponding S0 state energies calculated at these relaxed S1 geometries. As Fig. 16(a) shows, the PEC for the 1CT state is barrierless and forms a CI with the S0 PES at extended RN–H. The calculated energy of this MECI is below the VEE of any of the ‘bright’ LE states. Radiationless transfer to and from this 1CT state thus provides a route by which base pairs initially excited to a 1ππ* LE state can return to the S0 state and, following a ground state H atom transfer, reform the starting base pair structure – thereby constituting another possible route to photostability. Perun et al.170 also calculated PECs for the corresponding 1ππ* LE and 1CT states for what is the most stable A–T conformer in the gas phase (characterised by H-bonds between N9–H on A and the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2 carbonyl group on T, and between N1–H on T and N3 on A). The PEC for the 1CT state in this conformer, assuming RN9–H as the driving coordinate, was found at significantly higher energies than the analogous 1CT state in the WC A–T base pair and, crucially, not to form a CI with the S0 PES.
C2 carbonyl group on T, and between N1–H on T and N3 on A). The PEC for the 1CT state in this conformer, assuming RN9–H as the driving coordinate, was found at significantly higher energies than the analogous 1CT state in the WC A–T base pair and, crucially, not to form a CI with the S0 PES.
Equivalent studies of the WC G–C base pair identified 1ππ* LE states centred on both G and C and a low-lying 1ππ* CT state formed by exciting a G-centred π electron to a C-centred π* orbital.173–177 As Fig. 16(b) shows, the PEC for this 1CT state calculated using RN1–H as the driving coordinate exhibits a CI with the S0 state. Thus, as with the WC A–T base pair, theory suggests EDPT via a 1CT state could provide an efficient non-radiative route to the S0 state after UV excitation of the WC G–C base pair. Again, the calculated PEC for the analogous 1CT state in the most stable (gas phase) conformer of G–C (i.e. with H-bonds between N1–H on G and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C2 on C, and between the C6
C2 on C, and between the C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on G and N1–H on C) using the RN1–H bond in G as the driving coordinate shows no analogous CI with the S0 PES.178
O on G and N1–H on C) using the RN1–H bond in G as the driving coordinate shows no analogous CI with the S0 PES.178
Analyses of REMPI spectra of jet-cooled samples of many DNA/RNA base pair structures serve to reinforce these conclusions. The long wavelength UV absorption spectra of those structures assigned as WC conformers are broad and diffuse, whereas the corresponding spectra of all other conformers investigated to date exhibit sharp structure.179,180 Solution phase ultrafast transient absorption studies of the WC G–C base pair confirm that the spectral breadth is attributable to the short excited state lifetime.181,182 All such observations serve to support suggestions that the WC variants of the A–T and G–C base pairs have a special status, distinguished by the availability of uniquely efficient 1CT state based deactivation mechanisms that minimise the opportunity for deleterious UV photoinduced chemistry. However, we also note that solution phase transient absorption studies of the WC G–C base pair (in chloroform) suggest a small yield of products arising from a double proton transfer process, wherein one of the H atoms in the amine group attached to the C4 atom in the C moiety in the 1CT state is transferred to the O atom in the C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in G.183 The net effect of the second (reverse) H atom transfer is to return population to the S0 state, albeit in a different tautomeric form than the starting WC base pair.
O group in G.183 The net effect of the second (reverse) H atom transfer is to return population to the S0 state, albeit in a different tautomeric form than the starting WC base pair.
The importance of out-of-plane ring deformations in facilitating IC in N containing heterocycles has been highlighted earlier in this Article. Thus, as for the isolated bases (Fig. 9 and 11), we have used a LIIC approach to calculate PECs along the Qoop coordinate leading to the MECI between an excited state and the S0 state of the WC A–T base pair. As Fig. 17 shows, the potential energies of the first few singlet excited states generally increase with out-of-plane distortion. However, the PEC for one excited state in the energy range falls with increasing Qoop and exhibits a CI with the S0 state. Inspecting the various orbital promotions (shown in the ESI,† Fig. S33), we find that the former excited states all arise via π* ← n or π* ← π electron promotions localised on one of the chromophores, whereas the one excited state that displays a very different Qoop dependence is the first formed by a π* ← π excitation involving orbitals that are delocalised (DE) over the entire base pair. The calculated energy of this 1ππ*(DE)/S0 MECI (∼7 eV) is much higher than that of the 1ππ*/S0 MECIs identified in the corresponding Qoop coordinate in the isolated A or T bases. This difference can be attributed to the inter-base H-bonding interactions, which serve to restrain the out-of-plane distortion of the individual bases. This digression provides one illustration of the subtle, yet complex, consequences of non-covalent interactions on the relative efficacies of different non-radiative decay pathways: out-of-plane deformations are pivotal in promoting the non-radiative decay of the excited states of individual DNA bases yet, once non-covalently linked as a WC base pair, the dominant relaxation pathway is generally via CIs located along the EDPT coordinate.
A thorough survey of even a subset of the many other instances where non-covalent interactions have been implicated in the non-radiative decay of stacked base pairs, and single and double stranded DNA, is outside the scope of this article. But we end by highlighting one example that serves to illustrate the potential for competition between base-pairing and base-stacking interactions. Early reports of a longer lived (∼100 ps) decay component in transient absorption studies following UV excitation of single and double-stranded oligonucleotides composed of A and T units184,185 have since been extended and generalised to include other bases and short segments of both single and double stranded DNA.186–193 The long lived component is now attributed to excited states arising as a result of photo-induced intrastrand electron transfer (ET), whereby a π electron from one base in a stack of nucleosides or base pairs is promoted to a π* orbital of an adjacent base in the same stack (i.e. electron hole pair creation in neighbouring stacked DNA bases). The intrastrand ET could then drive interstrand proton transfer from, or to, the base paired with, respectively, the radical anion and the radical cation formed in the ET process – yielding rare tautomers of the original base pair which then decay on a sub-nanosecond timescale. The similarities between this and the classic EDPT mechanism invoked to explain the ultrafast non-radiative decay of electronic excitation in isolated base pairs170,172 (illustrated in Fig. 16) are self-evident. So, too, is the difference. The electron and proton implicated in these long-lived excited states originate from neighbouring bases in a stack (rather than the same base in a single base pair).
The PECs shown in Fig. 18 illustrate aspects of this behaviour. We start with a G–C base pair within a stack, and assume that the C carries a negative charge as a result of photo-induced ET from a neighbouring base (in the strand). This we model as a radical-anion G–C− pair. Fig. 18(a) shows the relaxed PEC along RN1–H (the same driving coordinate as in Fig. 16(b)) for the ground state neutral G–C base pair (solid black points), and PECs for the G–C− radical-anion pair both at the relevant neutral S0 minimum energy geometries and after allowing all other nuclear degrees of freedom to relax to the minimum energy geometry of its doublet ground (D0) state (depicted by open and filled red points, respectively). The inset at the upper right shows the calculated neutral (S0) and radical-anion (D0) PECs along a LIIC linking the minimum energy geometry of the ground state neutral (at RN1–H = 1.038 Å) and the minimum energy configuration of the radical-anion at RN1–H = 1.238 Å. The calculated PECs show a low energy CI in the RN1–H coordinate associated with proton transfer from the G to the C−. This process, which the main figure shows to be mildly exothermic, is illustrated on the structure shown at the lower right. Fig. 18(b) shows the corresponding PECs for the neutral A–T base pair and the A–T− radical-anion along the same RN–H coordinate as used in Fig. 16(a). The LIIC used in the upper insert in this case spans the range 1.0 ≤ RN–H ≤ 1.2 Å. Though qualitatively similar to those for the G–C base pair (Fig. 18(a)), these PECs show significant differences: the energy of the neutral base pair at its equilibrium geometry is always below that of the radical-anion, and the potential energy of the relaxed D0 PEC if anything increases with increasing RN–H. These model calculations thus suggest no driving force for proton transfer from A to T−. Such contrasting propensities for proton transfer, from G to C− but not from A to T−, accord well with the recent time-resolved vibrational spectroscopy measurements of Zhang et al.193
4. Conclusions
This Perspective Article reports a systematic analysis of possible non-radiative decay mechanisms following UV photoexcitation of families of nitrogen containing heterocycles. The availability of non-radiative decay mechanisms by which photoexcited molecules can funnel back to their ground state, without experiencing potentially deleterious chemical transformation, is fundamental to molecular photostability. Photostability requires that the rate of internal conversion to the original ground state configuration far exceeds that of any competing population loss process (e.g. fluorescence, isomerisations, reaction, bond fission, etc.). To achieve such ‘ultrafast’ non-radiative decay from an excited state PES to the ground state, the molecule must be able to sample regions of conical intersection between the PESs.194The present investigation relies on use of electronic structure methods, complemented by a large body of pre-existing experimental and theoretical literature. Starting from phenol and indole, we have explored the structural and photophysical consequences of progressive incorporation of N atoms into the ring structure. Specific unicyclic systems investigated include selected hydroxypyridines, hydroxypyrimidines and the DNA bases T, U and C. Amongst the bicyclic systems, we have focussed on two azaindoles, purine, A and G. Having identified and rationalised specific photophysical trends in these families of molecular prototypes, we then extended the study to consider some of the additional richness and complexity with regard to excited state non-radiative decay that can arise via non-covalent interactions – in, for example, ribonucleosides, base pairs and stacked bases and base pairs. CIs are shown to be pervasive, but the forms of nuclear distortion required to access the CIs of lowest energy (and thus facilitate non-radiative decay) are seen to be sensitively dependent on the starting molecule, and its immediate environment. Nonetheless, as we now summarise, certain guiding trends and principles clearly emerge from the study.
Addressing first the isolated molecular prototypes, N incorporation is shown to result in a progressive relative stabilisation of the ground state keto-tautomer (cf. the enol-form), consistent with the observed dominance of keto-tautomers in the DNA/RNA nucleobases, ribonucleosides and WC base pairs. Focussing now on excited states, O–H bond fission is a common population loss process following UV photoexcitation of phenols.9,17,19,25 However, the present work shows that incorporating even one N atom affords sufficient stabilisation of the 1ππ* PES towards a 1ππ*/S0 CI in an out-of-plane deformation coordinate that IC via such prefulvenic distorted structures becomes the dominant (and efficient) decay route following UV excitation of the N-containing unicycles. Such predictions accord with the available excited state lifetime data. The simplest bicyclic system considered in this work, indole, already contains one N atom. This atom is in the 5-membered ring, however, and the lowest energy 1ππ*/S0 CI in the Qoop coordinate is calculated to lie well above the S1(1Lb) ← S0 origin – consistent with prior observations of fluorescence from electronically excited state indole molecules.195 The present calculations show that the addition of at least one more N atom, in the 6-membered ring, is necessary in order to modify the topography of the 1ππ* PES sufficiently that distortion along Qoop to a CI with the S0 PES becomes a barrierless, exothermic non-radiative decay pathway. Such trends, identified in both the unicyclic and bicyclic families, are consistent with the well-documented ultrafast excited state decay (and the photostability) of the isolated DNA(RNA) bases T(U), C, A and G.
Understanding the excited state decay dynamics of isolated nucleobases is a necessary but, as the latter part of this Perspective Article shows, not a sufficient prerequisite for unravelling the dominant non-radiative decay mechanism(s) operating when the base is a part of a larger chemical structure. In the nucleosides, for example, one or more heteroatoms in the base may experience H-bonding interactions with OH groups of the sugar backbone. From the perspective of the respective bases, such interactions are predicted to have only a minor perturbative effect on the 1ππ* PES leading to the 1ππ*/S0 MECI along Qoop. But the existence of such H-bonds can also promote new, alternative non-radiative decay pathways wherein charge-transfer character induced by the photexcitation causes an H atom to migrate from the sugar to one of the heteroatoms of the base. The present calculations hint that – particularly in the cases of adenosine, guanosine and cytidine – such an EDPT mechanism could be a competitive route for channeling excited state population back to the S0 state via a CI in the relevant O–H⋯N coordinate.
H-bonds are central to base pair formation, and have a profound effect on the photophysics of electronically excited base pairs. Isolated base-pairs decay non-radiatively, on an ultrafast timescale. But this decay is not via1ππ*/S0 CIs in the Qoop coordinate as in the individual bases; the energies of such CIs are substantially raised in the base-pairs by the interbase H-bonds that restrain the out-of-plane distortion of either component base. Rather, the minimum energy decay pathway following UV excitation involves interbase charge-transfer, and subsequent motion through a low energy CI in the EDPT coordinate associated with extending the N6–H bond in A (in the WC A–T base pair) or N1–H in G (in the WC G–C base pair). The WC structures are not the lowest energy conformers of the isolated base pairs in the gas phase. But equivalent calculations for these minimum energy conformers of A–T and G–C reveal no similar low energy 1CT/S0 CI in the EDPT coordinate, encouraging suggestions that the WC variants are special – by virtue of their having a particularly efficient 1ππ* CT state based deactivation mechanism that minimises the opportunity for potentially UV induced photodamage.
This survey ends with a note of caution. Not only do studies of the isolated bases fail to reveal key aspects of the excited state photophysics displayed by the WC base pairs, but theoretical studies of the isolated base pairs also fail to account for all aspects of the photophysics exhibited by stacks of base pairs. Specifically, several recent experiments identify a longer lifetime component within the excited state decay which theory can explain by invoking a role for photoinduced intrastrand electron transfer, which drives subsequent interstrand proton transfer within a base pair – thereby returning the system to the ground state of (a variant) of the original base pair.183–192 Given the evident influence of non-covalent interactions, it is worth reiterating that the calculations reported in this Perspective are all performed under isolated molecule conditions. Many prior absorption studies have shown that solvation affects the absorption spectra (and thus the relative energies of the excited states) of such systems. However, none of the recent time-resolved experiments or theoretical investigations suggest that solvent interactions change the following fundamental conclusion: the excited states of individual DNA/RNA bases, of the WC base pairs, and of assemblies of such base pairs, in the gas phase or in solution, all have access to a sufficiently diverse range of low energy non-radiative decay pathways to ensure rapid and efficient repopulation of the ground state, and thus photostability.
Acknowledgements
The Bristol based authors are grateful to EPSRC for the award of a Programme Grant (EP/L005913) and to colleagues Prof. Andrew Orr-Ewing and Dr Katharina Röttger for their invaluable feedback on an earlier draft of this manuscript. TNVK is grateful for the award of a TUM University Foundation Fellowship. Additional outputs that underpin data reported in this paper have been placed in the University of Bristol's research data repository and can be accessed using the following DOI: http://10.5523/bris.16bza2y6ry2iz14jsuovowv592.References
- A. L. Sobolewski and W. Domcke, Chem. Phys., 2000, 259, 181–191 CrossRef CAS.
- A. L. Sobolewski, W. Domcke, C. Dedonder-Lardeux and C. Jouvet, Phys. Chem. Chem. Phys., 2002, 4, 1093–1100 RSC.
- M. N. R. Ashfold, B. Cronin, A. L. Devine, R. N. Dixon and M. G. D. Nix, Science, 2006, 321, 1637–1640 CrossRef PubMed.
- K.-L. Han and G.-Z. He, J. Photochem. Photobiol., C, 2007, 8, 55–66 CrossRef CAS.
- M. N. R. Ashfold, G. A. King, D. Murdock, M. G. D. Nix, T. A. A. Oliver and A. G. Sage, Phys. Chem. Chem. Phys., 2010, 12, 1218–1238 RSC.
- W. Domcke and D. R. Yarkony, Annu. Rev. Phys. Chem., 2012, 63, 325–352 CrossRef CAS PubMed.
- G. M. Roberts and V. G. Stavros, Chem. Sci., 2014, 5, 1698–1722 RSC.
- Z. Lan, W. Domcke, V. Vallet, A. L. Sobolewski and S. Mahapatra, J. Chem. Phys., 2005, 122, 224315 CrossRef PubMed.
- M. G. D. Nix, A. L. Devine, B. Cronin, R. N. Dixon and M. N. R. Ashfold, J. Chem. Phys., 2006, 125, 133318 CrossRef PubMed.
- A. L. Devine, M. G. D. Nix, B. Cronin and M. N. R. Ashfold, Phys. Chem. Chem. Phys., 2007, 9, 3749–3762 RSC.
- G. A. King, A. L. Devine, M. G. D. Nix, D. E. Kelly and M. N. R. Ashfold, Phys. Chem. Chem. Phys., 2008, 10, 6417–6429 RSC.
- M. N. R. Ashfold, A. L. Devine, R. N. Dixon, G. A. King, M. G D. Nix and T. A. A. Oliver, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12701–12706 CrossRef CAS PubMed.
- A. Iqbal, L.-J. Pegg and V. G. Stavros, J. Phys. Chem. A, 2008, 112, 9531–9534 CrossRef CAS PubMed.
- O. P. J. Vieuxmaire, Z. Lan, A. L. Sobolewski and W. Domcke, J. Chem. Phys., 2008, 129, 224307 CrossRef PubMed.
- G. A. King, T. A. A. Oliver, M. G. D. Nix and M. N. R. Ashfold, J. Phys. Chem. A, 2009, 113, 7984–7993 CrossRef CAS PubMed.
- G. A. Pino, A. N. Oldani, E. Marceca, M. Fujii, S.-I. Ishiuchi, M. Miyazaki, M. Broquier, C. Dedonder and C. Jouvet, J. Chem. Phys., 2010, 133, 124313 CrossRef CAS PubMed.
- R. N. Dixon, T. A. A. Oliver and M. N. R. Ashfold, J. Chem. Phys., 2011, 134, 194303 CrossRef PubMed.
- R. A. Livingstone, J. O. F. Thompson, M. Iljina, R. J. Donaldson, B. J. Sussman, M. J. Paterson and D. Townsend, J. Chem. Phys., 2012, 137, 184304 CrossRef PubMed.
- G. M. Roberts, A. S. Chatterley, J. D. Young and V. G. Stavros, J. Phys. Chem. Lett., 2012, 3, 348–352 CrossRef CAS PubMed.
- D. J. Hadden, G. M. Roberts, T. N. V. Karsili, M. N. R. Ashfold and V. G. Stavros, Phys. Chem. Chem. Phys., 2012, 14, 13415–13428 RSC.
- S. G. Ramesh and W. Domcke, Faraday Discuss., 2013, 163, 73–94 RSC.
- X. Xu, K. R. Yang and D. G. Truhlar, J. Chem. Theory Comput., 2013, 9, 3612–3625 CrossRef CAS PubMed.
- T. N. V. Karsili, A. M. Wenge, S. J. Harris, D. Murdock, J. N. Harvey, R. N. Dixon and M. N. R. Ashfold, Chem. Sci., 2013, 4, 2434–2446 RSC.
- S. Albert, P. Lerch, R. Prentner and M. Quack, Angew. Chem., Int. Ed., 2013, 52, 346–349 CrossRef CAS PubMed.
- T. N. V. Karsili, A. M. Wenge, B. Marchetti and M. N. R. Ashfold, Phys. Chem. Chem. Phys., 2014, 16, 588–598 RSC.
- X. Xu, J. Zheng, K. R. Yang and D. G. Truhlar, J. Am. Chem. Soc., 2014, 136, 16378–16386 CrossRef CAS PubMed.
- K. R. Yang, X. Xu, J. Zheng and D. G. Truhlar, Chem. Sci., 2014, 5, 4661–4680 RSC.
- S. J. Harris, T. N. V. Karsili, D. Murdock, T. A. A. Oliver, A. M. Wenge, D. K. Zaouris, M. N. R. Ashfold, J. N. Harvey, J. D. Few, S. Gowrie, G. Hancock, D. J. Hadden, G. M. Roberts, V. G. Stavros, G. Spighi, L. Poisson and B. Soep, J. Phys. Chem. A, 2015, 119, 6045–6056 CrossRef CAS PubMed.
- A. L. Sobolewski and W. Domcke, Chem. Phys. Lett., 1999, 315, 293–298 CrossRef CAS.
- B. C. Dian, A. Longarte and T. S. Zwier, J. Chem. Phys., 2003, 6, 2696–2706 CrossRef.
- H. Lippert, H.-H. Ritze, I. V. Hertel and W. Radloff, Chem. Phys. Lett., 2004, 398, 526–531 CrossRef CAS.
- M.-F. Lin, C.-M. Tseng, Y. T. Lee and C.-K. Ni, J. Chem. Phys., 2005, 123, 124303 CrossRef PubMed.
- M. G. D. Nix, A. L. Devine, B. Cronin and M. N. R. Ashfold, Phys. Chem. Chem. Phys., 2006, 8, 2610–2618 RSC.
- C. Brand, J. Küpper, D. W. Pratt, W. L. Meerts, D. Krügler, J. Tatchen and M. Schmitt, Phys. Chem. Chem. Phys., 2010, 12, 4968–4979 RSC.
- A. Iqbal and V. G. Stavros, J. Phys. Chem. A, 2010, 114, 68–72 CrossRef CAS PubMed.
- T. A. A. Oliver, G. A. King and M. N. R. Ashfold, Phys. Chem. Chem. Phys., 2011, 13, 14646–14662 RSC.
- R. Livingstone, O. Schalk, A. E. Boguslavskiy, G. Wu, L. T. Bergendahl, A. Stolow, M. J. Paterson and D. Townsend, J. Chem. Phys., 2011, 135, 194307 CrossRef PubMed.
- A. Giussani, M. Merchán, D. Roca-Sanjuán and R. Lindh, J. Chem. Theory Comput., 2011, 7, 4088–4096 CrossRef CAS PubMed.
- R. Montero, A. P. Conde, V. Ovejas, F. Castaño and A. Longarte, J. Phys. Chem. A, 2012, 116, 2698–2703 CrossRef CAS PubMed.
- B. O. Roos, P.-Å. Malmqvist, V. Molina, L. Serrano-Andrés and M. Merchán, J. Chem. Phys., 2002, 116, 7526–7536 CrossRef CAS.
- F. B. C. Machado and E. R. Davidson, J. Chem. Phys., 1992, 97, 1881–1891 CrossRef CAS.
- L. Serrano-Andrés, M. P. Fülscher, B. O. Roos and M. Merchán, J. Phys. Chem., 1996, 100, 6484–6491 CrossRef.
- M. Barbatti, H. Lischka, S. Salzmann and C. M. Marian, J. Chem. Phys., 2009, 130, 034305 CrossRef PubMed.
- D. Voet, W. B. Gratzer, R. A. Cox and P. Doty, Biopolymers, 1963, 1, 193–208 CrossRef CAS.
- P. R. Callis, Annu. Rev. Phys. Chem., 1983, 34, 329–357 CrossRef CAS.
- C. E. Crespo-Hernández, B. Cohen, P. M. Hare and B. Kohler, Chem. Rev., 2004, 104, 1977–2019 CrossRef PubMed.
- B. Cohen, C. E. Crespo-Hernández, P. M. Hare and B. Kohler, in Femtochemistry and Femtobiology: Ultrafast Events in Molecular Science, ed. M. Martin and J. T. Hynes, Elsevier, 2004, pp. 463–470 Search PubMed.
- A. L. Sobolewski and W. Domcke, Europhys. News, 2006, 37, 20–23 CrossRef CAS.
- C. T. Middleton, K. de la Harpe, C. Su, Y. K. Law, C. E. Crespo-Hernández and B. Kohler, Annu. Rev. Phys. Chem., 2009, 60, 217–239 CrossRef CAS PubMed.
- A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2010, 12, 4897–4898 RSC.
- T. Gustavsson, R. Improta and D. Markovitsi, J. Phys. Chem. Lett., 2010, 1, 2025–2030 CrossRef CAS.
- D. Markovitsi, T. Gustavsson and I. Vayá, J. Phys. Chem. Lett., 2010, 1, 3271–3276 CrossRef CAS.
- K. Kleinermanns, D. Nachtigallová and M. S. de Vries, Int. Rev. Phys. Chem., 2013, 32, 308–342 CrossRef CAS.
- H. Kang, K. T. Lee, B. Jung, Y. J. Ko and S. K. Kim, J. Am. Chem. Soc., 2002, 124, 12958–12959 CrossRef CAS PubMed.
- S. Ullrich, T. Schultz, M. Z. Zgierski and A. Stolow, Phys. Chem. Chem. Phys., 2004, 6, 2796–2801 RSC.
- C. Canuel, M. Mons, F. Piuzzi, B. Tardivel, I. Dimicoli and M. Elhanine, J. Chem. Phys., 2005, 122, 074316 CrossRef PubMed.
- K. Kosma, C. Schröter, E. Samoylova, I. V. Hertel and T. Schultz, J. Am. Chem. Soc., 2009, 131, 16939–16943 CrossRef CAS PubMed.
- J.-W. Ho, H.-C. Yen, W.-K. Chou, C.-N. Weng, L.-H. Cheng, H.-Q. Shi, S.-H. Lai and P.-Y. Cheng, J. Phys. Chem. A, 2011, 115, 8406–8418 CrossRef CAS PubMed.
- M. Kotur, T. C. Weinacht, C. Zhou, K. A. Kistler and S. Matsika, J. Chem. Phys., 2011, 134, 184309 CrossRef PubMed.
- S. Lobsiger, M. A. Trachsel, H.-M. Frey and S. Leutwyler, J. Phys. Chem. A, 2013, 117, 6106–6115 CrossRef CAS PubMed.
- H. Kang, B. Jung and S. K. Kim, J. Chem. Phys., 2003, 118, 6717–6719 CrossRef CAS.
- S. Ullrich, T. Schultz, M. Z. Zgierski and A. Stolow, J. Am. Chem. Soc., 2004, 126, 2262–2263 CrossRef CAS PubMed.
- H. Satzger, D. Townsend, M. Z. Zgierski, S. Patchkovskii, S. Ullrich and A. Stolow, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10196–10201 CrossRef CAS PubMed.
- C. Z. Bisgaard, H. Satzger, S. Ullrich and A. Stolow, ChemPhysChem, 2009, 10, 101–110 CrossRef CAS PubMed.
- H. Kang, J. Chang, S. H. Lee, T. K. Ahn, N. J. Kim and S. K. Kim, J. Chem. Phys., 2010, 133, 154311 CrossRef PubMed.
- D. N. Nikogosyan, D. Angelov, B. Soep and L. Lindqvist, Chem. Phys. Lett., 1996, 252, 322–326 CrossRef.
- A. Reuther, H. Iglev, R. Laenen and A. Laubereau, Chem. Phys. Lett., 2000, 325, 360–368 CrossRef CAS.
- J.-M. L. Pecourt, J. Peon and B. Kohler, J. Am. Chem. Soc., 2000, 122, 9348–9349 CrossRef CAS.
- T. Gustavsson, A. Sharonov and D. Markovitsi, Chem. Phys. Lett., 2002, 351, 195–200 CrossRef CAS.
- T. Gustavsson, A. Sharonov, D. Onidas and D. Markovitsi, Chem. Phys. Lett., 2002, 356, 49–54 CrossRef CAS.
- A. Sharonov, T. Gustavsson, V. Carré, E. Renault and D. Markovitsi, Chem. Phys. Lett., 2003, 380, 173–180 CrossRef CAS.
- B. Cohen, P. Hare and B. Kohler, J. Am. Chem. Soc., 2003, 125, 13594–13601 CrossRef CAS PubMed.
- R. J. Malone, A. M. Miller and B. Kohler, Photochem. Photobiol., 2003, 77, 158–164 CrossRef CAS PubMed.
- T. Gustavsson, Á. Bányász, E. Lazzarotto, D. Markovitsi, G. Scalmani, M. J. Frisch, V. Barone and R. Improta, J. Am. Chem. Soc., 2006, 128, 607–619 CrossRef CAS PubMed.
- T. Gustavsson, N. Sarkar, E. Lazzarotto, D. Markovitsi and R. Improta, Chem. Phys. Lett., 2006, 429, 551–557 CrossRef CAS.
- G. M. Roberts, H. J. B. Marroux, M. P. Grubb, M. N. R. Ashfold and A. J. Orr-Ewing, J. Phys. Chem. A, 2014, 118, 11211–11225 CrossRef CAS PubMed.
- F. Buchner, A. Nakayama, S. Yamazaki, H.-H. Ritze and A. Lübcke, J. Am. Chem. Soc., 2015, 137, 2931–2938 CrossRef CAS PubMed.
- S. Matsika, J. Phys. Chem. A, 2004, 108, 7584–7590 CrossRef CAS.
- M. Z. Zgierski, S. Patchkovskii, T. Fujiwara and E. C. Lim, J. Phys. Chem. A, 2005, 109, 9384–9387 CrossRef CAS PubMed.
- S. Perun, A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2006, 110, 13238–13244 CrossRef CAS PubMed.
- N. Ismail, L. Blancafort, M. Olivucci, B. Kohler and M. A. Robb, J. Am. Chem. Soc., 2002, 124, 6818–6819 CrossRef CAS PubMed.
- L. Blancafort, B. Cohen, P. M. Hare, B. Kohler and M. A. Robb, J. Phys. Chem. A, 2005, 109, 4431–4436 CrossRef CAS PubMed.
- K. A. Kistler and S. Matsika, J. Phys. Chem. A, 2007, 111, 2650–2661 CrossRef CAS PubMed.
- L. Blancafort, Photochem. Photobiol., 2007, 83, 603–610 CrossRef CAS PubMed.
- M. Merchán, R. González-Luque, T. Climent, L. Serrano-Andrés, E. Rodríguez, M. Reguero and D. Peláez, J. Phys. Chem. B, 2006, 110, 26471–26476 CrossRef PubMed.
- G. Zechmann and M. Barbatti, J. Phys. Chem. A, 2008, 112, 8273–8279 CrossRef CAS PubMed.
- D. Asturiol, B. Lasorne, M. A. Robb and L. Blancafort, J. Phys. Chem. A, 2009, 113, 10211–10218 CrossRef CAS PubMed.
- S. Yamazaki, W. Domcke and A. L. Sobolewski, J. Phys. Chem. A, 2008, 112, 11965–11968 CrossRef CAS PubMed.
- Z. Lan, E. Fabiano and W. Thiel, ChemPhysChem, 2009, 10, 1225–1229 CrossRef CAS PubMed.
- K. A. Kistler and S. Matsika, Phys. Chem. Chem. Phys., 2010, 12, 5024–5031 RSC.
- M. Barbatti, A. J. A. Aquino, J. J. Szymczak, D. Nachtigallova, P. Hobza and H. Lischka, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 21453–21458 CrossRef CAS PubMed.
- V. B. Delchev, A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2010, 12, 5007–5015 RSC.
- M. Barbatti, J. J. Szymczak, A. J. A. Aquino, D. Nachtigallova and H. Lischka, J. Chem. Phys., 2011, 134, 014304 CrossRef PubMed.
- D. Nachtigallová, A. J. A. Aquino, J. J. Szymczak, M. Barbatti, P. Hobza and H. Lischka, J. Phys. Chem. A, 2011, 115, 5247–5255 CrossRef PubMed.
- C. M. Marian, J. Chem. Phys., 2005, 122, 104314 CrossRef PubMed.
- S. Perun, A. L. Sobolewski and W. Domcke, J. Am. Chem. Soc., 2005, 127, 6257–6265 CrossRef CAS PubMed.
- L. Serrano-Andrés, M. Merchán and A. C. Borin, Chem. – Eur. J., 2006, 12, 6559–6571 CrossRef PubMed.
- M. Barbatti and H. Lischka, J. Am. Chem. Soc., 2008, 130, 6831–6839 CrossRef CAS PubMed.
- I. Conti, M. Garavelli and G. Orlandi, J. Am. Chem. Soc., 2009, 131, 16108–16118 CrossRef CAS PubMed.
- M. Barbatti, Z. Lan, R. Crespo-Otero, J. J. Szymczak, H. Lischka and W. Thiel, J. Chem. Phys., 2012, 137, 22A503 CrossRef PubMed.
- J. Petersen, M. Wohlgemuth, B. Sellner, V. Bonačić-Koutecký, H. Lischka and R. Mitrić, Phys. Chem. Chem. Phys., 2012, 14, 4687–4694 RSC.
- B. P. Fingerhut, K. E. Dorfman and S. Mukamel, J. Phys. Chem. Lett., 2013, 4, 1933–1942 CrossRef CAS PubMed.
- C. G. Triandafillou and S. Matsika, J. Phys. Chem. A, 2013, 117, 12165–12174 CrossRef CAS PubMed.
- F. Plasser, R. Crespo-Otero, M. Pederzoli, J. Pittner, H. Lischka and M. Barbatti, J. Chem. Theory Comput., 2014, 10, 1395–1405 CrossRef CAS PubMed.
- C. M. Marian, J. Phys. Chem. A, 2007, 111, 1545–1553 CrossRef CAS PubMed.
- S. Yamazaki and W. Domcke, J. Phys. Chem. A, 2008, 112, 7090–7097 CrossRef CAS PubMed.
- L. Serrano-Andrés, M. Merchán and A. C. Borin, J. Am. Chem. Soc., 2008, 130, 2473–2484 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennuchi and G. Petersson, et al., Gaussian 09, revision B.01, Gaussian Inc., Wallingford, CT, 2010 Search PubMed.
- T. H. Dunning, Jr., J. Chem. Phys., 1989, 90, 1007–1023 CrossRef.
- W. J. Hehre, R. F. Stewart and J. A. Pople, J. Chem. Phys., 1969, 51, 2657–2664 CrossRef CAS.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, et al., MOLPRO, version 2010.1, a package of ab initio programs, University of Cardiff, Cardiff, UK, 2010, see http://www.molpro.net Search PubMed.
- Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007, available from http://www.turbomole.com.
- L. D. Hatherley, R. D. Brown, P. D. Godfrey, A. P. Pierlot, W. Caminati, D. Damiani, S. Melandri and L. B. Favero, J. Phys. Chem., 1993, 97, 46–51 CrossRef CAS.
- R. H. Cox and A. A. Bother-By, J. Phys. Chem., 1969, 73, 2465–2468 CrossRef CAS.
- M. Kuzuya, A. Noguchi and T. Okuda, J. Chem. Soc., Chem. Commun., 1984, 100, 435–436 RSC.
- R. Sanchez, B. M. Giuliano, S. Melandri, L. B. Favero and W. Caminati, J. Am. Chem. Soc., 2007, 129, 6287–6290 CrossRef CAS PubMed.
- B. M. Giuliano, V. Feyer, K. C. Prince, M. Coreno, L. Evangelisti, S. Melandri and W. Caminati, J. Phys. Chem. A, 2010, 114, 12725–12730 CrossRef CAS PubMed.
- R. D. Brown, P. D. Godfrey, D. McNaughton and A. P. Pierlot, J. Am. Chem. Soc., 1988, 110, 2329–2330 CrossRef CAS.
- R. D. Brown, P. D. Godfrey, D. McNaughton and A. P. Pierlot, J. Am. Chem. Soc., 1989, 111, 2308–2310 CrossRef CAS.
- E. Nir, M. Muller, L. I. Grace and M. S. de Vries, Chem. Phys. Lett., 2002, 355, 59–64 CrossRef CAS.
- E. Nir, I. Hünig, K. Kleinermanns and M. S. de Vries, Phys. Chem. Chem. Phys., 2003, 5, 4780–4785 RSC.
- V. Feyer, O. Plekan, R. Richter, M. Coreno, G. Vall-Ilosera, K. C. Prince, A. B. Trofimov, I. L. Zatseva, T. E. Moskovskaya, E. V. Gromov and J. Schirmer, J. Phys. Chem. A, 2009, 113, 5736–5742 CrossRef CAS PubMed.
- M. Szczesniak, K. Szczepaniak, J. S. Kwiatkowski, K. KuBulat and W. B. Person, J. Am. Chem. Soc., 1988, 110, 8319–8330 CrossRef CAS.
- G. Bazsó, G. Tarczay, G. Fogarasi and P. G. Szalay, Phys. Chem. Chem. Phys., 2011, 13, 6799–6807 RSC.
- M. Dreyfus, O. Bensaude, G. Dodin and J. E. Dubois, J. Am. Chem. Soc., 1976, 98, 6338–6349 CrossRef CAS PubMed.
- C. Colominas, F. J. Luque and M. Orozco, J. Am. Chem. Soc., 1996, 118, 6811–6821 CrossRef CAS.
- R. Kobayashi, J. Phys. Chem., 1998, 102, 10813–10817 CrossRef CAS.
- S. A. Trygubenko, T. V. Bogdan, M. Rueda, M. Orozco, F. J. Luque, J. Sponer, P. Slavıcek and P. Hobza, Phys. Chem. Chem. Phys., 2002, 4, 4192–4203 RSC.
- M. Hanus, M. Kabeláč, J. Rejnek, F. Ryjáček and P. Hobza, J. Phys. Chem. B, 2004, 108, 2087–2097 CrossRef CAS.
- J. Lin, C. Yu, S. Peng, I. Akiyama, K. Li, L. K. Lee and P. R. LeBreton, J. Am. Chem. Soc., 1980, 102, 4627–4631 CrossRef CAS.
- R. D. Brown, P. D. Godfrey, D. McNaughton and A. P. Pierlot, Chem. Phys. Lett., 1989, 156, 61–63 CrossRef CAS.
- C. Plützer, E. Nir, M. S. de Vries and K. Kleinermanns, Phys. Chem. Chem. Phys., 2001, 3, 5466–5469 RSC.
- C. Plützer and K. Kleinermanns, Phys. Chem. Chem. Phys., 2002, 4, 4877–4882 RSC.
- M. J. Nowak, L. Lapinski, J. S. Kwiatkowski and J. Leszczyński, J. Phys. Chem., 1996, 100, 3527–3534 CrossRef CAS.
- M. Dreyfus, G. Dodin, O. Bensaude and J. E. Dubois, J. Am. Chem. Soc., 1975, 97, 2369–2376 CrossRef CAS PubMed.
- M.-T. Chenon, R. J. Pugmire, D. M. Grant, R. P. Panzica and L. B. Townsend, J. Am. Chem. Soc., 1975, 97, 4636–4642 CrossRef CAS PubMed.
- N. C. Gonnella, H. Nakanishi, J. B. Holtwick, D. S. Horowitz, K. Kanamori, N. J. Leonard and J. D. Roberts, J. Am. Chem. Soc., 1983, 105, 2050–2055 CrossRef CAS.
- A. Laxer, D. T. Major, H. E. Gottlieb and B. Fischer, J. Org. Chem., 2001, 66, 5463–5481 CrossRef CAS PubMed.
- S. K. Mishra, M. K. Shukla and P. C. Mishra, Spectrochim. Acta, Part A, 2000, 56, 1355–1384 CrossRef.
- L. M. Salter and G. M. Chaban, J. Phys. Chem. A, 2002, 106, 4251–4256 CrossRef CAS.
- T.-K. Ha, H.-J. Keller, R. Gunde and H.-H. Gunthard, J. Phys. Chem. A, 1999, 103, 6612–6623 CrossRef CAS.
- K. Szczepaniak and M. Szczesniak, J. Mol. Struct., 1987, 156, 29–42 CrossRef CAS.
- F. Piuzzi, M. Mons, I. Dimicoli, B. Tardivel and Q. Zhao, Chem. Phys., 2001, 270, 205–214 CrossRef CAS.
- M. Mons, I. Dimicoli, F. Piuzzi, B. Tardivel and M. Elhanine, J. Phys. Chem. A, 2002, 106, 5088–5094 CrossRef CAS.
- M. Mons, F. Piuzzi, I. Dimicoli, L. Gorb and J. Leszczynski, J. Phys. Chem. A, 2006, 110, 10921–10924 CrossRef CAS PubMed.
- K. Seefeld, R. Brause, T. Häber and K. Kleinermanns, J. Phys. Chem. A, 2007, 111, 6217–6221 CrossRef CAS PubMed.
- M. Hanus, F. Ryjáček, M. Kabelaáč, T. Kubař, T. V. Bogdan, S. A. Trygubenko and P. Hobza, J. Am. Chem. Soc., 2003, 125, 7678–7688 CrossRef CAS PubMed.
- L. Gorb, A. Kaczmarek, A. Gorb, A. J. Sadlej and J. Leszczynski, J. Phys. Chem. B, 2005, 109, 13770–13776 CrossRef CAS PubMed.
- S. Kato, J. Chem. Phys., 1988, 88, 3045–3056 CrossRef CAS.
- I. J. Palmer, I. N. Ragazos, F. Bernardi, M. Olivucci and M. A. Robb, J. Am. Chem. Soc., 1993, 115, 673–682 CrossRef CAS.
- A. L. Sobolewski, C. Woywod and W. Domcke, J. Chem. Phys., 1993, 98, 5627–5641 CrossRef CAS.
- F. Zhang, Y.-J. Ai, Y. Luo and W.-H. Fang, J. Chem. Phys., 2009, 130, 144315 CrossRef PubMed.
- A. L. Sobolewski, Chem. Phys. Lett., 1993, 211, 293–299 CrossRef CAS.
- A. L. Sobolewski and L. Adamowicz, J. Phys. Chem., 1996, 100, 3933–3941 CrossRef CAS.
- H. Ozeki, M. C. R. Cockett, K. Okayama, M. Takahashi and K. Kimura, J. Phys. Chem., 1995, 99, 8608–8612 CrossRef CAS.
- D. Lee, S. J. Baek, K.-W. Choi, Y. S. Choi and S. K. Kim, Bull. Korean Chem. Soc., 2002, 23, 277–280 CrossRef CAS.
- A. Held, B. B. Champagne and D. W. Pratt, J. Chem. Phys., 1991, 95, 8732–8743 CrossRef CAS.
- M. R. Nimlos, D. F. Kelley and E. R. Bernstein, J. Phys. Chem., 1989, 93, 643–651 CrossRef CAS.
- S. Maeda, Y. Harabuchi, T. Taketsugu and K. Morokuma, J. Phys. Chem. A, 2014, 118, 12050–12058 CrossRef CAS PubMed.
- D. Nachtigallová, H. Lischka, J. J. Szymczak, M. Barbatti, P. Hobza, Z. Gengeliczki, G. Pino, M. P. Callahane and M. S. de Vries, Phys. Chem. Chem. Phys., 2010, 12, 4924–4933 RSC.
- M. G. D. Nix, A. L. Devine, B. Cronin and M. N. R. Ashfold, J. Chem. Phys., 2007, 126, 124312 CrossRef PubMed.
- A. Z. Britten and G. Lockwood, Spectrochim. Acta, Part A, 1976, 32, 1335–1338 CrossRef.
- V. Ludwig, Z. M. da Costa, M. S. do Amaral, A. C. Borin, S. Canuto and L. Serrano-Andrés, Chem. Phys. Lett., 2010, 492, 164–169 CrossRef CAS.
- J. Hager and S. C. Wallace, J. Phys. Chem., 1983, 87, 2121–2127 CrossRef CAS.
- Y. Huang and M. Sulkes, Chem. Phys. Lett., 1996, 254, 242–248 CrossRef CAS.
- M. S. de Vries, Top. Curr. Chem., 2014, 355, 33–56 CrossRef.
- H. Asami, K. Yagi, M. Ohba, S.-H. Urashima and H. Saigusa, Chem. Phys., 2013, 419, 84–89 CrossRef CAS.
- D. Tuna, A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2014, 118, 122–127 CrossRef CAS PubMed.
- M. Z. Zgierski and S. Alavi, Chem. Phys. Lett., 2006, 426, 398–404 CrossRef CAS.
- S. De Camillis, J. Miles, G. Alexander, O. Ghafur, I. D. Williams, D. Townsend and J. B. Greenwood, Phys. Chem. Chem. Phys., 2015, 17, 23643–23650 RSC.
- S. Perun, A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2006, 110, 9031–9038 CrossRef CAS PubMed.
- J. P. Gobbo, V. Saurí, D. Roca-Sanjuán, L. Serrano-Andrés, M. Merchán and A. C. Borin, J. Phys. Chem. B, 2012, 116, 4089–4097 CrossRef CAS PubMed.
- A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2004, 6, 2763–2771 RSC.
- P. R. Markwick and N. L. Doltsinis, J. Chem. Phys., 2007, 126, 175102 CrossRef PubMed.
- G. Groenhof, L. V. Schäfer, M. Boggio-Pasqua, M. Goette, H. Grubmüller and M. A. Robb, J. Am. Chem. Soc., 2007, 129, 6812–6819 CrossRef CAS PubMed.
- V. Saurí, J. P. Gobbo, J. J. Serrano-Pérez, M. Lundberg, P. B. Coto, L. Serrano-Andrés, A. C. Borin, R. Lindh, M. Merchán and D. Roca-Sanjuán, J. Chem. Theory Comput., 2013, 9, 481–496 CrossRef PubMed.
- S. Yamazaki and T. Taketsugu, Phys. Chem. Chem. Phys., 2012, 14, 8866–8877 RSC.
- A. L. Sobolewski, W. Domcke and C. Hättig, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17903–17906 CrossRef CAS PubMed.
- E. Nir, C. Plützer, K. Kleinermanns and M. S. de Vries, Eur. Phys. J. D, 2002, 20, 317–329 CrossRef CAS.
- A. Abo-Riziq, L. Grace, E. Nir, M. Kabelac, P. Hobza and M. S. de Vries, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 20–23 CrossRef CAS PubMed.
- N. K. Schwalb and F. Temps, J. Am. Chem. Soc., 2007, 129, 9272–9273 CrossRef CAS PubMed.
- K. Röttger and F. Temps, in Ultrafast Phenomena XIX, ed. I. Yamanouchi, S. Cundiff and R. de Vivie Riedle, 2015, vol. 162, pp. 514–516 Search PubMed.
- K. Röttger, H. J. B. Marroux, M. P. Grubb, P. M. Coulter, H. Böhnke, A. S. Henderson, M. C. Galan, F. Temps, A. J. Orr-Ewing and G. M. Roberts, Angew. Chem., Int. Ed., 2015, 54, 14719–14722 CrossRef PubMed.
- C. Crespo-Hernández, B. Cohen and B. Kohler, Nature, 2005, 436, 1141–1144 CrossRef PubMed.
- W.-M. Kwok, C. Ma and D. L. Phillips, J. Am. Chem. Soc., 2006, 128, 11894–11905 CrossRef CAS PubMed.
- T. Takaya, C. Su, K. de la Harpe, C. E. Crespo-Hernández and B. Kohler, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 10285–10290 CrossRef CAS PubMed.
- K. de la Harpe and B. Kohler, J. Phys. Chem. Lett., 2011, 2, 133–138 CrossRef CAS.
- I. Vaya, T. Gustavsson, T. Douki, Y. Berlin and D. Markovitsi, J. Am. Chem. Soc., 2012, 134, 11366–11368 CrossRef CAS PubMed.
- G. W. Doorley, M. Wojdyla, G. W. Watson, M. Towrie, A. W. Parker, J. M. Kelly and S. J. Quinn, J. Phys. Chem. Lett., 2013, 4, 2739–2744 CrossRef CAS.
- C. Su, C. T. Middleton and B. Kohler, J. Phys. Chem. B, 2012, 116, 10266–10274 CrossRef CAS PubMed.
- J. Chen, A. K. Thazhathveetil, F. D. Lewis and B. Kohler, J. Am. Chem. Soc., 2013, 136, 10290–10293 CrossRef PubMed.
- D. B. Bucher, A. Schlueter, T. Carell and W. Zinth, Angew. Chem., Int. Ed., 2014, 55, 11366–11369 CrossRef PubMed.
- Y. Zhang, K. de la Harpe, A. A. Beckstead, R. Improta and B. Kohler, J. Am. Chem. Soc., 2015, 137, 7059–7062 CrossRef CAS PubMed.
- W. Domcke, D. R. Yarkony and H. Koppel, Conical Intersections: Theory, Computation and Experiment, vol. 17 of Advances Series in Physical Chemistry, 2011, and references therein Search PubMed.
- D. M. Sammeth, S. Yan, L. H. Spangler and P. R. Callis, J. Phys. Chem., 1990, 94, 7340–7342 CrossRef CAS.
- Y. Matsuda, T. Ebata and N. Mikami, J. Chem. Phys., 1999, 110, 8397–8407 CrossRef CAS.
- Y. Matsuda, T. Ebata and N. Mikami, J. Chem. Phys., 2000, 113, 573–580 CrossRef CAS.
- L. B. Clark, G. G. Peschel and I. Tinoco, Jr., J. Phys. Chem., 1965, 69, 3615–3618 CrossRef CAS.
- H. De Voe and I. Tinoco, Jr., J. Mol. Biol., 1962, 4, 500–517 CrossRef CAS.
- B. J. Fender, D. M. Sammeth and P. R. Callis, Chem. Phys. Lett., 1995, 239, 31–37 CrossRef CAS.
- K. Fuke, H. Yoshiuchi and K. Kaya, J. Phys. Chem., 1984, 88, 5840–5844 CrossRef CAS.
- S. K. Kim and E. R. Bernstein, J. Phys. Chem., 1990, 94, 3531–3539 CrossRef CAS.
- C. Kang, J. T. Yi and D. W. Pratt, J. Chem. Phys., 2005, 123, 094306 CrossRef PubMed.
- K. Sakota and H. Sekiya, J. Phys. Chem. A, 2009, 113, 2663–2665 CrossRef CAS PubMed.
- M. Schneider, T. Hain and I. Fischer, ChemPhysChem, 2009, 10, 634–636 CrossRef CAS PubMed.
- N. J. Kim, G. Jeong, Y. S. Kim, J. Sung, S. K. Kim and Y. D. Park, J. Chem. Phys., 2000, 113, 10051–10055 CrossRef CAS.
- E. Nir, C. Janzen, P. Imhof, K. Kleinermanns and M. S. de Vries, J. Chem. Phys., 2001, 115, 4604–4611 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: More details regarding the computational methods used; PECs along the relevant Qoop coordinate for all molecules displayed in Fig. 1 and 2; depictions of the active space orbitals used for the CASSCF and CASPT2 calculations; dominant orbital excitations that contribute to the first three singlet excited states of all molecules featured in Fig. 1–3 and 4(a); and PECs for the ground and first few singlet excited states of adenosine along Qoop calculated at the ADC(2)/cc-pVDZ level of theory. See DOI: 10.1039/c6cp00165c |
| This journal is © the Owner Societies 2016 |