Ultra-thin and porous MoSe2 nanosheets: facile preparation and enhanced electrocatalytic activity towards the hydrogen evolution reaction†
Zhouyue
Lei
,
Shengjie
Xu
and
Peiyi
Wu
*
State Key Laboratory of Molecular Engineering of Polymers, Collaborative Innovation Center of Polymers and Polymer Composite Materials, Department of Macromolecular Science and Laboratory for Advanced Materials, Fudan University, Shanghai 200433, China. E-mail: peiyiwu@fudan.edu.cn
First published on 12th November 2015
Abstract
In this study, ultra-thin and porous molybdenum selenide (MoSe2) nanosheets were prepared through a modified liquid exfoliation method as efficient electrocatalysts for the hydrogen evolution reaction (HER). This novel structure enables the exposure of more catalytically active sites and moreover maintains effective electron transport, resulting in a small peak potential of ∼75 mV as well as long-term durability. In addition, due to the facile and economical preparation method as well as its eco-friendly synthetic conditions, this study provides a high-performance HER catalyst with promising commercial application prospects.
Hydrogen has attracted increasing research interests due to its great potential in sustainable energy harvesting and conversion field, and the fact that hydrogen energy conversion technologies are mainly limited by the development of highly active, acid-stable and inexpensive catalysts.1 Recently, two-dimensional (2D) transition metal dichalcogenides (TMDs) have attracted more attention due to their potential electrocatalytic activity towards the hydrogen evolution reaction (HER).2–6 In general, it is predicted by theory that ultra-thin TMD nanostructures are favored for increasing surface areas with more exposed planes, and the edges of TMD nanosheets have higher activity towards the HER.7 Therefore, if TMDs based catalysts are to realize their potential, there are two intractable problems that demand some ingenious solutions, i.e., achieving a facile method to obtain ultra-thin nanosheets and optimizing their nanostructures with high active site density.
Conventionally, 2D nanosheets are prepared by either top-down micromechanical cleavage8 or bottom-up chemical-vapor deposition.9,10 These methods enable the production of single- or few-layer nanosheets, but they are unsuitable in practical applications on a large scale, which usually needs large quantities of materials in a processable form.11 Although the massive preparation of TMDs nanosheets can be achieved via the Li-intercalation12 and liquid exfoliation13,14 methods, these strategies also suffer from drawbacks, where it is especially difficult to further tailor the morphology of TMDs. The Li-intercalation strategy needs extensive cleaning to get rid of impurities15,16 and always sacrifices the pristine semiconducting properties of TMDs bulk crystals due to the structural destruction during Li intercalation.16–18 As for the traditional liquid-phase exfoliation technique, it is in favor of the scalable production of TMDs nanosheets and liquid-suspended nanosheets can be directly processed into films. However, unfortunately in this scalable process, the sonic energy and the interactions between the TMDs and the solvents are usually insufficient to thoroughly exfoliate the crystals,11 and it is accompanied with the disadvantage of low exfoliation efficiency, i.e., long sonication time and thick nanosheets that are about several dozen nanometers. Overall, the liquid-phase exfoliation technique is the most appropriate method, but it is urgent to improve the efficiency of the exfoliation procedure and further tailor TMDs structures.
As for optimizing the HER catalytic activities of TMDs, there have been intensive efforts devoted to synthesizing TMDs nanostructures with a high density of active edge sites such as vertically aligned layers to expose the active edges of the TMDs,19 controllable disorder ultrathin molybdenum sulfide (MoS2) nanosheets with oxygen incorporation,20 macroporous molybdenum selenide (MoSe2) films21 and unique stack structure of MoS2 nanosheets.7 All the abovementioned strategies suggest increasing active sites play a crucial role in higher HER activity, thus this inspires us to postulate that ultra-thin and porous TMDs nanosheets with abundant active edge sites might be a very promising candidate for highly active HER catalysts, which can replace traditional Pt noble metal catalysts.
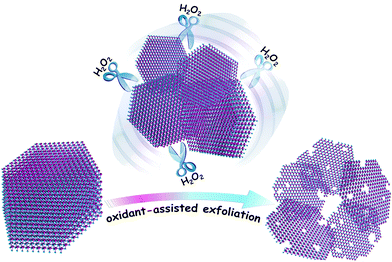
Herein, to realize the facile preparation of superior HER electrocatalysts with an optimized nanostructure, a modified liquid exfoliation method is proposed, that is, an appropriate oxidant such as H2O2 is utilized to assist the exfoliation of bulk TMDs nanosheets. When H2O2 is employed during the exfoliation of bulk MoSe2 flakes, even in the low-boiling-point solvent isopropyl alcohol (IPA), interestingly, the bulk MoSe2 flakes are easily incised and exfoliated into a desirable ultra-thin and porous structure, which is probably ascribed to the oxidation and etching effect of H2O2. These ultra-thin and porous MoSe2 nanosheets possess a high edge/basal ratio and exhibit excellent HER activity with a low overpotential of about 75 mV and long-term durability, which render them highly competitive with commercial Pt-based catalysts.
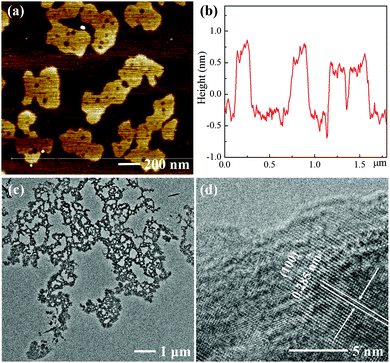
As shown in Scheme 1, bulk MoSe2 flakes are exfoliated and incised into ultra-thin and porous nanosheets with the assistance of H2O2. Atomic force microscopy (AFM) images show that the MoSe2 nanosheets are almost single- or double-layer with the thickness of 1–1.5 nm, which indicates their highly exfoliated nature, and their average lateral size is several hundred nanometers (Fig. 1). However, the layer thickness of the MoSe2 flakes exfoliated in IPA exceeds a few tens of nanometers, as shown in Fig. S1b (ESI†), which is probably due to the mismatched surface energies of IPA and TMDs.13 Even in the case of MoSe2 nanosheets exfoliated in better solvents with strong exfoliating ability for TMDs such as N-methyl-2-pyrrolidone (NMP),13,22 it is also intractable to exfoliate the nanosheets into single- or double-layer and further tailor their morphologies (Fig. S1c, ESI†). Nevertheless, the height profile of the MoSe2 nanosheets sharply decreases in the presence of H2O2, and more importantly the morphology of these ultra-thin MoSe2 nanosheets is significantly different from those exfoliated from common solvents. As a consequence of the H2O2 etching effect, the nanosheets are tailored into an optimized structure for the HER, in which plenty of pores and irregular lateral edges significantly improve the edge/basal ratio of the nanosheets and thus enhance their active surface areas with a higher catalytic active site density. Transmission electron microscope (TEM) images also confirm the porous and defect-rich morphology, as shown in Fig. 1c. The lattice fringe is 0.285 nm in the high resolution TEM (HRTEM) images, which corresponds to the (100) face of the MoSe2 crystal.23 In addition, field emission scanning electron microscope (FESEM) images and element mapping results further confirm the morphology and successful preparation of the ultra-thin and porous MoSe2 nanosheets, as shown in Fig. S2 (ESI†), although the presence of oxygen element might suggest the slight oxidation of some Mo edges, and this will be further discussed in the XPS results. Moreover, we carefully measured the concentration of the ultra-thin and porous MoSe2 nanosheets, which is about 0.7 mg mL−1, and therefore the yield is 7% (the initial amount of MoSe2/IPA was 10 mg mL−1). The concentration and yield of the ultra-thin and porous MoSe2 nanosheets are comparable to or even better than some previous reports on the liquid exfoliation of TMDs,22,24,25 thus indicating the high efficiency of this modified method.
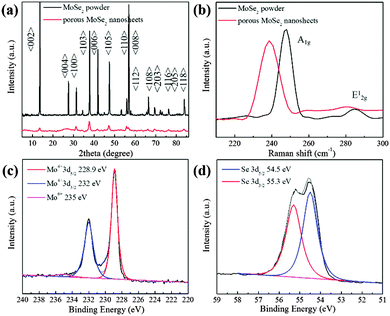
The crystal structure of the ultra-thin and porous nanosheets was further systematically investigated via X-ray powder diffraction (XRD) measurements and Raman spectroscopy. According to Fig. 2a, several main peaks at 13.7°, 37.8°, 47.5°, 55.8° and 56.7° are assigned to the (002), (103), (105), (110) and (008) faces of hexagonal 2H-MoSe2 (JCPDF (65-3481)). Compared with raw MoSe2, the (002) peak is clearly broadened, which indicates the highly exfoliated nature of the MoSe2 nanosheets. This is well consistent with the previous results of TEM and AFM, which also confirm the strong exfoliating ability of this mixing solvent system with H2O2 assistance. Moreover, the well-matched peaks suggest that the highly-exfoliated porous nanosheets still retain the pristine structure of 2H-MoSe2. This is also confirmed in a typical UV-vis spectrum of the porous MoSe2 nanosheets, as shown in Fig. S3 (ESI†). There are four characteristic absorption bands observed, which are in good agreement with previously reported studies, and these indicate no disturbance of the intrinsic MoSe2 crystal structure.26,27 Raman analysis is also applied to identify such layered materials. The Raman mode A1g of bulk MoSe2 is identified at 247.3 cm−1, whereas the A1g mode of the ultra-thin and porous MoSe2 nanosheets is identified at 238.6 cm−1. Such a redshift suggests the successful exfoliation of bulk MoSe2 and the few-layer structure of the porous MoSe2 nanosheets.28 Moreover, the broad and relatively weak in-plane (E12g) mode located at around 280 cm−1 indicates a high intensity ratio between the A1g and E12g modes, which is probably ascribed to the few-layer structure and relatively weak layer–layer interactions in the porous MoSe2 nanosheets.29 This also confirms the highly exfoliated features of the as-prepared MoSe2 nanosheets.
The chemical state of the porous nanosheets was further investigated via X-ray photoelectron spectroscopy (XPS). The peaks located at 228.9 (Mo4+ 3d5/2) and 232 eV (Mo4+ 3d3/2) confirm that molybdenum is basically in the Mo(IV) state, and the small and broad peak of Mo6+ (235 eV) is probably ascribed to the slight oxidation of some Mo edges, which should result from the oxidization of Mo(IV) in the presence of H2O2. The peaks identified at 55.3 and 54.5 eV in the high-resolution spectrum of Se are generally attributed to Se 3d3/2 and Se 3d5/2, respectively.30,31 These results further confirm that the as-prepared porous nanosheets mainly are MoSe2.
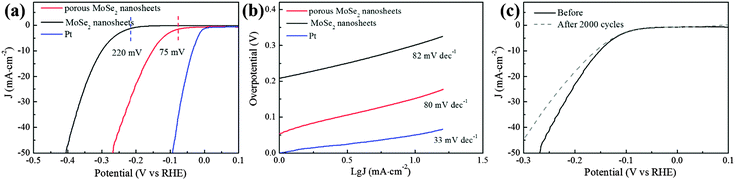
As a typical application, the ultra-thin and porous MoSe2 nanosheets were explored as electrocatalysts for the HER. HER activity was measured using the standard three electrode electrochemical configurations in N2 saturated 0.5 M H2SO4 electrolyte with a scan rate of 50 mV s−1.22,32 For comparison, few-layer MoSe2 nanosheets and commercial Pt (20 wt% Pt/C) catalysts were also included. As expected, the ultra-thin and porous MoSe2 nanosheets have better HER activity than the few-layer MoSe2 nanosheets (Fig. 3a). The onset potential manifests a significant positive shift to ∼75 mV, which is much better than few-layer MoSe2 nanosheets. Moreover, the overpotential of the porous MoSe2 nanosheets required for clear hydrogen evolution (−10 mA cm−2) is ∼150 mV, which is significantly lower than that of few-layer MoSe2 nanosheets (∼300 mV). Moreover, we have summarized the HER performance of the TMDs nanosheet (film) catalysts in Fig. S4 and Tables S1–S4 (ESI†), wherein it is obvious that the ultra-thin and porous MoSe2 nanosheets possess impressive HER activity among the state-of-the-art TMDs nanosheet (film) catalysts.
As an intrinsic property of electrocatalyst materials, the Tafel slope (Fig. 3b), which is associated with the rate-limiting step of the HER, has also been derived from Tafel plots, wherein their linear portions fit well with the Tafel equation (η = b × log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a, where j is the current density and b is the Tafel slope). As shown in Fig. 3b, the Tafel slope of commercial Pt (20 wt% Pt/C) is ∼33 mV dec−1, which is close to that reported in previous studies; this suggests that the HER on a Pt surface proceeds through the Volmer–Tafel mechanism and the Tafel reaction is the rate-limiting step at low overpotentials.33,34 Although the reaction mechanism on MoSe2 remained inconclusive, the Tafel slope of the porous MoSe2 nanosheets is 80 mV dec−1, which is similar to the MoSe2 nanosheets (82 mV dec−1) and comparable to the previous reports;30 this suggests a relative fast enhancement of the HER rate with the increase of overpotential.35
j + a, where j is the current density and b is the Tafel slope). As shown in Fig. 3b, the Tafel slope of commercial Pt (20 wt% Pt/C) is ∼33 mV dec−1, which is close to that reported in previous studies; this suggests that the HER on a Pt surface proceeds through the Volmer–Tafel mechanism and the Tafel reaction is the rate-limiting step at low overpotentials.33,34 Although the reaction mechanism on MoSe2 remained inconclusive, the Tafel slope of the porous MoSe2 nanosheets is 80 mV dec−1, which is similar to the MoSe2 nanosheets (82 mV dec−1) and comparable to the previous reports;30 this suggests a relative fast enhancement of the HER rate with the increase of overpotential.35
In addition to the onset potential and Tafel slope, the stability of electrocatalysts, as a key factor of catalytic activity, was also evaluated, as shown in Fig. 3c. The polarization curves before and after 1000 cycles are almost identical, and especially the onset potential is almost unchanged, indicating the good stability of the catalysts in a long-term electrochemical process.
To throw some light on the impressive HER performance of these ultra-thin and porous MoSe2 nanosheets, we further calculated their electrochemical active surface areas and investigated the electrochemical impedance spectra of the ultra-thin and porous MoSe2 nanosheets and few-layer MoSe2 nanosheets exfoliated in NMP. The estimation of the electrochemical active surface areas of the catalysts was conducted by measurement of the electrochemical double-layer capacitance in the potential region with no faradaic response using a simple cyclic voltammetry (CV) method.32,36,37 The CV results suggest that the electrochemical active surface area value for the ultra-thin and porous MoSe2 nanosheets is enhanced more than five-fold compared with that for the few-layer MoSe2 nanosheets, as shown in Fig. S5 and summarized in Table S5 (ESI†). Moreover, because the morphology characterization from the AFM and TEM images and structural analysis from the XRD and Raman results consistently confirm their highly exfoliated nature and porous defect-rich structure, this obvious increase in surface area is reasonable. Moreover, according to the EIS results in Fig. S6 (ESI†), the electrode kinetics of the ultra-thin porous MoSe2 nanosheets is similar to that of few-layer MoSe2 nanosheets, in which the charge transfer resistance (Rct) of the porous MoSe2 nanosheets is 25 Ω, which is slightly smaller than that of MoSe2 nanosheets (30 Ω), thus suggesting an even smaller charge transfer resistance in the optimized porous structure. Therefore, the impressive HER performance of the porous MoSe2 nanosheets is mainly attributed to two factors. On the one hand, it originates from the optimized structure of the ultra-thin and porous MoSe2 nanosheets. The porous structure results in a higher electrochemical active surface area and thus leads to a higher ratio of active edges and basal planes with more active edge sites.7 Thereby, the exposed surface area of the active sites contributes to the higher HER activity. On the other hand, the remaining continuous flat nanosheets also provide effective electrical contact between the electrode and the active sites. As confirmed in EIS results (Fig. S6, ESI†), the electrode kinetics of the porous MoSe2 nanosheets is slightly faster than that of the MoSe2 nanosheets.
In summary, we have developed a modified liquid exfoliation method to prepare ultra-thin and porous MoSe2 nanosheets. With the assistance of H2O2, bulk MoSe2 is easily exfoliated into an ultra-thin and porous structure. This facilitates the exposure of the active sites of the nanosheets and moreover the lamellar structure maintains effective electron transfer between the electrode and the catalytic sites. The resulting products exhibit impressive HER catalytic activity with a small onset potential of ∼75 mV, a Tafel slope of 80 mV dec−1, as well as long-term durability, which is even comparable to Pt-based HER catalysts. In addition, considering the facile and low-cost procedure, the ultra-thin and porous MoSe2 nanosheets is proposed to be a promising candidate for commercial HER catalysts in the future.
We gratefully acknowledge the financial support from the National Science Foundation of China (NSFC) (No. 21274030, 51473038).
Notes and references
- P. D. Tran and J. Barber, Phys. Chem. Chem. Phys., 2012, 14, 13772–13784 RSC.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- X. Huang, Z. Zeng and H. Zhang, Chem. Soc. Rev., 2013, 42, 1934–1946 RSC.
- T. Liu, C. Wang, X. Gu, H. Gong, L. Cheng, X. Shi, L. Feng, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 3433–3440 CrossRef CAS PubMed.
- H. Tang, K. Dou, C.-C. Kaun, Q. Kuang and S. Yang, J. Mater. Chem. A, 2014, 2, 360–364 CAS.
- U. Gupta, B. Naidu, U. Maitra, A. Singh, S. N. Shirodkar, U. V. Waghmare and C. Rao, APL Mater., 2014, 2, 092802 CrossRef.
- Z. Wu, B. Fang, Z. Wang, C. Wang, Z. Liu, F. Liu, W. Wang, A. Alfantazi, D. Wang and D. P. Wilkinson, ACS Catal., 2013, 3, 2101–2107 CrossRef CAS.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- A. Reina, X. Jia, J. Ho, D. Nezich, H. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, Nano Lett., 2009, 9, 30–35 CrossRef CAS PubMed.
- Y. Zhan, Z. Liu, S. Najmaei, P. M. Ajayan and J. Lou, Small, 2012, 8, 966–971 CrossRef CAS PubMed.
- D. Hanlon, C. Backes, T. M. Higgins, M. Hughes, A. O'Neill, P. King, N. McEvoy, G. S. Duesberg, B. Mendoza Sanchez, H. Pettersson, V. Nicolosi and J. N. Coleman, Chem. Mater., 2014, 26, 1751–1763 CrossRef CAS.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- A. O'Neill, U. Khan and J. N. Coleman, Chem. Mater., 2012, 24, 2414–2421 CrossRef.
- G. S. Bang, K. W. Nam, J. Y. Kim, J. Shin, J. W. Choi and S.-Y. Choi, ACS Appl. Mater. Interfaces, 2014, 6, 7084–7089 CAS.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Nano Lett., 2011, 11, 5111–5116 CrossRef CAS PubMed.
- V. Stengl and J. Henych, Nanoscale, 2013, 5, 3387–3394 RSC.
- J. Heising and M. G. Kanatzidis, J. Am. Chem. Soc., 1999, 121, 638–643 CrossRef CAS.
- D. Kong, H. Wang, J. J. Cha, M. Pasta, K. J. Koski, J. Yao and Y. Cui, Nano Lett., 2013, 13, 1341–1347 CrossRef CAS PubMed.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 17881–17888 CrossRef CAS PubMed.
- F. H. Saadi, A. I. Carim, J. M. Velazquez, J. H. Baricuatro, C. C. L. McCrory, M. P. Soriaga and N. S. Lewis, ACS Catal., 2014, 4, 2866–2873 CrossRef CAS.
- S. Xu, D. Li and P. Wu, Adv. Funct. Mater., 2015, 25, 1127–1136 CrossRef CAS.
- S. Mao, Z. Wen, S. Ci, X. Guo, K. K. Ostrikov and J. Chen, Small, 2014, 11, 414–419 CrossRef PubMed.
- G. Zhao, S. Han, A. Wang, Y. Wu, M. Zhao, Z. Wang and X. Hao, Adv. Funct. Mater., 2015, 25, 5292–5299 CrossRef CAS.
- E. Varrla, C. Backes, K. R. Paton, A. Harvey, Z. Gholamvand, J. McCauley and J. N. Coleman, Chem. Mater., 2015, 27, 1129–1139 CrossRef CAS.
- G. Cunningham, M. Lotya, C. S. Cucinotta, S. Sanvito, S. D. Bergin, R. Menzel, M. S. P. Shaffer and J. N. Coleman, ACS Nano, 2012, 6, 3468–3480 CrossRef CAS PubMed.
- Y. Shi, C. Hua, B. Li, X. Fang, C. Yao, Y. Zhang, Y.-S. Hu, Z. Wang, L. Chen, D. Zhao and G. D. Stucky, Adv. Funct. Mater., 2013, 23, 1832–1838 CrossRef CAS.
- J. Shaw, H. Zhou, Y. Chen, N. Weiss, Y. Liu, Y. Huang and X. Duan, Nano Res., 2014, 7, 511–517 CrossRef CAS.
- S. Tongay, J. Zhou, C. Ataca, K. Lo, T. S. Matthews, J. Li, J. C. Grossman and J. Wu, Nano Lett., 2012, 12, 5576–5580 CrossRef CAS PubMed.
- H. Wang, D. Kong, P. Johanes, J. J. Cha, G. Zheng, K. Yan, N. Liu and Y. Cui, Nano Lett., 2013, 13, 3426–3433 CrossRef CAS PubMed.
- G. W. Shim, K. Yoo, S.-B. Seo, J. Shin, D. Y. Jung, I.-S. Kang, C. W. Ahn, B. J. Cho and S.-Y. Choi, ACS Nano, 2014, 8, 6655–6662 CrossRef CAS PubMed.
- S. Xu, Z. Lei and P. Wu, J. Mater. Chem. A, 2015, 3, 16337–16347 CAS.
- J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807–5813 CrossRef CAS PubMed.
- Z. Wu, B. Fang, A. Bonakdarpour, A. Sun, D. P. Wilkinson and D. Wang, Appl. Catal., B, 2012, 125, 59–66 CrossRef CAS.
- D. Merki and X. Hu, Energy Environ. Sci., 2011, 4, 3878–3888 CAS.
- Y. Zheng, Y. Jiao, L. H. Li, T. Xing, Y. Chen, M. Jaroniec and S. Z. Qiao, ACS Nano, 2014, 8, 5290–5296 CrossRef CAS PubMed.
- J. Benson, M. Li, S. Wang, P. Wang and P. Papakonstantinou, ACS Appl. Mater. Interfaces, 2015, 7, 14113–14122 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: AFM images of MoSe2 nanosheets exfoliated in different solvents, FESEM images and element mapping images, UV-vis spectrum of the ultra-thin and porous MoSe2 nanosheets, cyclic voltammetry (CV) results, electrochemical impedance spectroscopy (EIS) results, and a comparison of the ultra-thin and porous MoSe2 nanosheets among state-of-the-art TMDs nanosheet (films) catalysts. See DOI: 10.1039/c5cp06483j |
| This journal is © the Owner Societies 2016 |