Open Access Article
Open Access ArticleChalcogens act as inner and outer heteroatoms in borane cages with possible consequences for σ-hole interactions
Jindřich
Fanfrlík
*a and
Drahomír
Hnyk
*b
aInstitute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, v.v.i. Flemingovo nam. 2, 166 10 Prague 6, Czech Republic. E-mail: fanfrlik@uochb.cas.cz
bInstitute of Inorganic Chemistry, Academy of Sciences of the Czech Republic, v.v.i. 250 68 Husinec-Řež, Czech Republic. E-mail: hnyk@iic.cas.cz
First published on 22nd September 2016
Abstract
Chalcogen bonds are a subset of σ-hole interactions and the number of their applications has been increasing rapidly. While chalcogen bonding in organic compounds has been intensively studied in the past decade, it is entirely new in boron cluster chemistry. In this paper, we review the remarkable ability of chalcogenated boron clusters to form strong chalcogen bonds. Chalcogen atoms have a unique property to be incorporated in a boron cluster both innerly and outerly. Various molecular shapes (e.g. icosahedral, bicapped-square antiprismatical) have been computationally analyzed and discussed. No general physical trend has been observed and, therefore, it is recommended to tackle each cluster individually.
Introduction
A chalcogen bond is an attractive interaction between an electrophilic (positive) region associated with a Group VI atom (abbreviated as Y atoms) in a molecular entity and a nucleophilic (negative) region in another, or the same, molecule. Chalcogen bonds have been intensively studied.1 Particularly, the S⋯O chalcogen bond has been attracting great attention because of its influence on protein structures2 and its importance for the biological activities of several organic molecules.3 Interestingly, the same chalcogen atom can simultaneously interact with both positive and negative sites. This ability has been explained in terms of regions of the positive electrostatic potential (ESP) on the outer surfaces of chalcogen atoms.4 These regions, called σ-holes, are located along the extensions of their existing covalent bonds.5 Chalcogen bonds are thus analogous to other σ-hole interactions, termed halogen bonds, which are of considerable importance in crystal engineering and drug design.6 A σ-hole is characterized by its magnitude (Vs,max) and spatial size.7Vs,max and the size increase with the atomic number of the chalcogen atom (from S to Te; analogous to that from Cl to I). They can likewise be modulated by changing the chemical environment, i.e. electron-withdrawing groups also increase the σ-hole. It has been demonstrated that the larger the Vs,max value, the stronger the halogen bond.7 A modulation of halogen bonds in protein–inhibitor complexes has been used to reduce the IC50 values accordingly.8Partial atomic charges on S atoms usually have negative values in simple organosulfur compounds where the S atoms are covalently bound to C and H atoms. Consequently, such S atoms have very small σ-holes. In some cases, the Vs,max can even have a negative value (e.g. Vs,max for H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S of −3.1 kcal mol−1).9,10 The Vs,max value increases with the introduction of electron-withdrawing groups as mentioned above (e.g. Vs,max for F2C
S of −3.1 kcal mol−1).9,10 The Vs,max value increases with the introduction of electron-withdrawing groups as mentioned above (e.g. Vs,max for F2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and S
S and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S of 12.6 and 16.0 kcal mol−1, respectively).10 On the other hand, partial atomic charges on S atoms have positive values when the S atom is bound to highly electronegative atoms (e.g. halogens). Consequently, the S atoms in such compounds have σ-holes with very large Vs,max values (e.g. Vs,max for SCl2, SCl4 and SF4 of 28.6, 38.3 and 51.5 kcal mol−1, respectively). Nziko and Schneier have theoretically studied the N⋯S chalcogen bond between SF4 and a series of amines. They have demonstrated that this chalcogen bond is very strong. The interaction energies ranged from −7 to −14 kcal mol−1 (for NH3 and trimethylamine, respectively).11 They also compared tetravalent SF4 with divalent SF2 and SHF. They found very small differences between the divalent and tetravalent S complexes.11
S of 12.6 and 16.0 kcal mol−1, respectively).10 On the other hand, partial atomic charges on S atoms have positive values when the S atom is bound to highly electronegative atoms (e.g. halogens). Consequently, the S atoms in such compounds have σ-holes with very large Vs,max values (e.g. Vs,max for SCl2, SCl4 and SF4 of 28.6, 38.3 and 51.5 kcal mol−1, respectively). Nziko and Schneier have theoretically studied the N⋯S chalcogen bond between SF4 and a series of amines. They have demonstrated that this chalcogen bond is very strong. The interaction energies ranged from −7 to −14 kcal mol−1 (for NH3 and trimethylamine, respectively).11 They also compared tetravalent SF4 with divalent SF2 and SHF. They found very small differences between the divalent and tetravalent S complexes.11
Interestingly, there are compounds with very large Vs,max values on the chalcogen atom even though the chalcogen atom is only bound to highly electropositive B atoms (i.e. the substituted boron hydrides that are described below). Closo-1-SB11H11 has five σ-holes with Vs,max values of 28.2 kcal mol−1.9Closo-1-SB11H11 has been shown to form strong complexes with trimethylamine and benzene (interaction energy of −6.5 and −6.3 kcal mol−1, respectively) in our computational study.12 As mentioned above, the strength of chalcogen bonds increases with the atomic number of the chalcogen. Closo-1-SeB11H11 thus forms even stronger chalcogen bonded complexes: the interaction energies of closo-1-SeB11H11 with trimethylamine and benzene are −8.1 and −7.0 kcal mol−1, respectively.12 These are very strong chalcogen bonds considering that the interaction energies of the benzene⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2, benzene⋯Se(CF3)2, trimethylamine⋯Se
CF2, benzene⋯Se(CF3)2, trimethylamine⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 and trimethylamine⋯Se(CF3)2 complexes possessing the chalcogen bond are −3.5, −4.5, −4.6 and −3.7 kcal mol−1, respectively.13
CF2 and trimethylamine⋯Se(CF3)2 complexes possessing the chalcogen bond are −3.5, −4.5, −4.6 and −3.7 kcal mol−1, respectively.13
Boron clusters
Boron atoms have a remarkable ability to form molecules of unlimited size by covalently bonding to themselves and other elements. Polyhedral boron clusters (boranes) exhibit an astonishing variety of stable molecular structures due to this ability.17 Boranes have a broad range of applications including radioactive waste extraction, nanotechnology and medicinal chemistry. Such a wide range of applications are enabled by the unique properties of boranes, which include high stability, low toxicity, hydrophobicity18 and the ability to form nonclassical noncovalent interactions19 such as dihydrogen bonds20 and σ-hole interactions.9 Although boron is the neighbor of carbon in the periodic table, their chemistries differ dramatically.21 Their geometries are not compatible even in the case of simple B2H6 and C2H6. B2H6, possessing the D2h symmetry, differs from the ethane-like structure of the D3d symmetry with two-center two-electron bond (2c–2e). Conceivably, B2H6 could be fitted to the electron diffraction data only if a hydrogen-bridge-based model with the so-called 3-center 2-electron bond (3c–2e) of the D2h symmetry were considered.22 Further experimental structural studies have led to the formulation of the unusual bonding principles in boron clusters (the concept multicenter bonding)23 by W. N. Lipscomb. An important class of boranes comprises closo-BnHn2− (n = 5–12) dianions. The systematic replacement of BH (formally neutral) vertices in them by a variety of chalcogens such as S2+, Se2+, or other heteroatoms (e.g. CH+), leads to a broad range of so-called heteroboranes.As opposed to the electronegativity concept, parent boron hydrides (boranes) are electron deficient and any heteroatom within a cluster framework (endo substituent) acts as if it were an electron donor. Consequently, the heterovertex (or a midpoint of two-heterovertex separation) is a center of a partial positive charge, as evidenced by interpreting experimental dipole moments. Indeed, it is the midpoint of the CC vector that is the center of the partial positive charge in closo-1,2-C2B10H12, which has been determined experimentally in an unambiguous way.24 The same applies to another closo system, closo-1-SB11H11, where the S atom bears the partially positive charge in the icosahedral cage.25 We have modeled the unsynthesized closo-1-OB11H11 compound26 in order to demonstrate the effect of a boron cluster framework for heteroatoms. Vs,max values were positive even on the highly electronegative O atom (Table 1).
| Molecule (symmetry) | V s,max HF/cc-pVDZ | q B3LYP/cc-pVTZ | α B3LYP/cc-pVTZ |
|---|---|---|---|
| a Trigonal bipyramidal shape dictated by the so-called quadruple average angle, see. ref. 11b. b A more stable form with Cs symmetry has 2 σ-holes with a Vs,max value of 19.8 kcal mol−1. c V s,max values were determined on the 0.001 a.u. molecular surface computed at the HF/cc-pVDZ level using the Gaussian 09,15 Molekel4.3 (ref. 16a and b) and WFA (wavefunction analysis)16c programs. It has been recently shown that larger basis sets are not needed for these purposes.16d d It was recently shown that the RESP,16e in contrast to NBO, represents a method of choice for heteroboranes, as NBO's closely correspond to the picture of localized bonds and lone pairs as basic units of the molecular structure. This is not true for delocalized heteroboranes.20 e α is the second-order tensor of the dipole polarizability; the reported average polarizability is given by 1/3 (αxx + αyy + αzz). | |||
H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (C2v) S (C2v) |
−3.1 (ref. 9) | −0.15 | 29.5 |
F2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (C2v) S (C2v) |
12.6 (ref. 9) | −0.13 | 30.7 |
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (D∞h) S (D∞h) |
16.0 (ref. 14) | 0.05 | 49.2 |
| SCl2 (C2v) | 2 × 28.6 | 0.07 | 42.4 |
| SCl4 (C2v)a | 2 × 38.3 | 0.38 | 87.3 |
| SF2 (C2v) | 2 × 45.5 | 0.23 | 18.9 |
| SF4 (C2v)a | 2 × 51.5 | 0.44 | 25.5 |
| Closo-1-SB4H4 (C3v) | 3 × 16.9 | 0.04 | 67.9 |
| Closo-1-SB5H5 (C4v) | 4 × 11.1 | 0.17 | 77.1 |
| Closo-1-SB9H9 (C4v) | 4 × 22.4 (ref. 12) | 0.19 | 122.1 |
| Closo-1-SB6H6 (C5v) | 5 × 12.3 | 0.17 | 88.4 |
| Closo-1-SB11H11 (C5v) | 5 × 28.2 (ref. 12) | 0.21 | 139.7 |
| Closo-1-OB11H11 (C5v) | 5 × 11.7 | −0.07 | 126.9 |
| Closo-1-SeB11H11 (C5v) | 5 × 29.5 (ref. 12) | 0.31 | 145.8 |
| Closo-1-SB7H7 (C6v)b | 6 × 10.4 | 0.06 | 101.3 |
| Closo-1-SB13H13 (C6v) | 6 × 22.9 | 0.15 | 165.7 |
| 12-F-closo-1-SB11H11 (C5v) | 5 × 29.2 (ref. 12) | 0.22 | 139.7 |
| 1,2-(SH)2-closo-1,2-C2B10H10 (C2v) | 16.0 | −0.10 | 169.2 |
| 1,2-(SeH)2-closo-1,2-C2B10H10 (C2v) | 20.4 | 0.01 | 181.7 |
Chalcogens and hydrogenated chalcogens in boron clusters
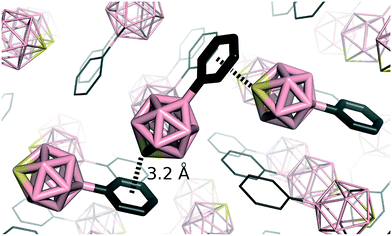
As mentioned above, BH vertices in closo-BnHn2− (n = 5–12) can be replaced by various heterovertices. Several parental heteroboranes with a chalcogen vertex have been synthesized, specifically closo-1-SB11H11,27closo-1-SeB11H11,28closo-1-SB9H9 (ref. 29) and closo-1-TeB11H11.30 All these neutral parent icosahedral heteroboranes have been geometrically characterized using gas-phase electron diffraction since their monocrystals are disordered, which prevents them from X-ray diffraction analyses. However, this is not the case of some exo-substituted heteroboranes. We have demonstrated the dominant role of chalcogen bonding in the crystal packing for 12-Ph-closo-1-SB11H10 (Fig. 2).9It would be very tempting to have some guidelines for the relations of physical properties of thiaboranes going from closo-SBnHn to closo-SBn+1Hn+1. In order to obtain deeper insight into thiaborane properties, we have analyzed the NMR chemical shifts and Vs,max values of various thiaboranes. The experimental observation related to a strong downfield 11B NMR chemical shift of the antipodal boron atom in closo-1-SB9H9 (a symmetry of C4v) and closo-1-SB11H11 (a symmetry of C5v) may, however, lead to unrealistic assumptions.27,29 Considering the Vs,max values and NMR shifts of closo-1-SB9H9 and closo-1-SB11H11 (22.4 and 28.2 kcal mol−1 (ref. 12) and 76 and 18 ppm,27,29 respectively), we would have expected a larger computed Vmax for the hypothetical closo-1-SB13H13 (a symmetry of C6v,31δ(11B(antipodal B13)) = −8.4 ppm) than for closo-1-SB11H11 because this downfield chemical shift decreases with the increasing number of the vertices. However, the Vs,max value of this hypothetical bicapped-hexagon-type antiprismatic thiaborane closo-1-SB13H13 is computed to be “only” 22.9 kcal mol−1. Conceivably, there is no relation between the electrostatic potential, i.e. the partial atomic charge on the S atom, and the 11B chemical shift of the B atom antipodally coupled with the S atom. The same applies to a series of hypothetical bipyramidal thiaboranes with symmetries of C3v,C4v, C5v, and C6v (see Table 1, the isoelectronic monocarbaboranes are experimentally available31c). These computational results further support the known fact that there are no relations as to the physical and chemical properties of closo-heteroboranes going from closo-YBnHn to closo-YBn+1Hn+1.32 Each cluster thus must be treated individually, which will also be true for future applications in crystal engineering.
Chalcogen atoms are able to act as both inner and outer heteroatoms in boranes. In the form of YH, they can substitute exopolyhedral hydrogens and in the form of Y BH vertices in them. As an example of exo-chalcogenated carboranes, we can list experimentally known 1,2-(SH)2-closo-1,2-C2B10H10,33 9,12-(SH)2-closo-1,2-C2B10H10,34 1,2-(SeH)2-closo-1,2-C2B10H10 (ref. 35) and still hypothetical 9,12-(SeH)2-closo-1,2-C2B10H10.36 The S atom of 9,12-(SH)2-closo-1,2-C2B10H10 is bound to the B vertex. It has a negative ESP surface and it does not have a σ-hole (see Fig. 1). Conversely, the S atom of 1,2-(SH)2-closo-1,2-C2B10H10 is bound to the C heterovertex of the carborane cage. It has a positive σ-hole along the extension of the S–H bond with the Vs,max value of 16.0 kcal mol−1. There is an even more positive ESP surface along the extension of the S–B bond, but it is impossible to determine its Vs,max value, because the ESP does not have a local maximum here. The ESP continuously becomes more positive towards the H atom (see Fig. 1). There is a clear analogy between carboranes with exopolyhedral chalcogen and halogen atoms. We have recently computed that the σ-hole on Br atoms of brominated carboranes could be highly positive but only if it were bound to a C vertex.12,37 On the other hand, the Vs,max on the Br atom had a negative value when the Br atom was bound to a B vertex. Interestingly, this observation is also true in dilute solutions of closo-1-Ph-2-X-1,2-C2B10H10 (for X = F, Cl, Br, I),38 where dipole moments were measured and subsequently interpreted by a simple vector algebra.38 The observed dipole-moment anomaly for X = Br, I in contrast to F, Cl could be accounted for only when an additional vector pointing from the outer surface of bromine and iodine (irrespective of the corresponding electronegativities) towards the negative part of the aromatic quadrupole moment was introduced, which confirmed the anisotropy of heavier halogen atoms.
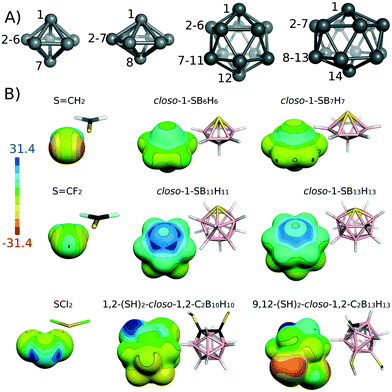 | ||
| Fig. 1 (A) The structure and numbering scheme of 7-, 8-, 12- and 14-vertex cages adopting the symmetries of C5v, C6v, C5v and C6v, respectively. (B) The molecular diagrams and electrostatic potential (ESP) on the 0.001 a.u. molecular surface computed at the HF/cc-pVDZ level using the Gaussian 09 (ref. 15) and Molekel4.3 (ref. 16a and b) programs for selected S-atom possessing molecules. Color range in kcal mol−1. | ||
A comparison of closo-YB11H11 with the 1,2-(YH)2-closo-1,2-C2B10H10 series suggests that the effect of the borane cages is more pronounced (i.e. higher Vs,max values) when the chalcogen atom is an endo substituent (see Table 1). This difference is the most significant when Y = O: the O atom of 1,2-(OH)2-closo-1,2-C2B10H10 has a negative ESP surface in contrast to closo-1-OB11H11, where we have computed a positive Vs,max value.
We have recently decomposed several interaction energies of chalcogen bonded complexes in order to establish the nature of chalcogen bonding.12 The electrostatic and dispersion terms were found to be systematically predominant. The strength of the electrostatic term is predetermined by the Vs,max value on the Y atom, which was discussed above. The strength of the dispersion energy is determined in terms of polarisability. The computed polarisabilities (Table 1) of the studied compounds are large and even comparable to halogenated carboranes.37
It should also be mentioned that the σ-hole interaction can play an important role in reaction mechanisms if chalcogen atoms are involved. We have recently modeled a synthetic route to 1,7-(SCN)2-closo-B12H102−. We have found that the σ-hole on the elongation of the S–S axis of (SNC)2 plays a role when approaching the closo-B12H122− anion.39
Conclusions
The chemistry of chalcogenated boranes is very broad. Chalcogen atoms can replace both BH vertices and exopolyhedral H atoms of boranes and heteroboranes. Due to the electro-deficiency of boron cages, any heterovertex (endo substituent) acts as an e− donor and becomes a center of a partial positive charge. Consequently, any chalcogen atom within a boron cluster framework and any heavier chalcogen atom bound to the C heterovertex of neutral carboranes have highly positive σ-holes. These compounds thus have a remarkable ability to form strong chalcogen bonds,40 which makes them attractive for many exciting applications in materials chemistry, crystal engineering as well as in other fields.Acknowledgements
This work has been supported by the research project RVO 61388963 of the Academy of Sciences of the Czech Republic. We acknowledge the financial support of the Czech Science Foundation (JF: P208/12/G016 and DH: 15-056775).Notes and references
- A. Bauza, T. J. Mooibroek and A. Frontera, ChemPhysChem, 2015, 16, 2496 CrossRef CAS PubMed.
- (a) M. Iwaoka, S. Takemoto, M. Okada and S. Tomoda, Chem. Lett., 2001, 132 CrossRef CAS; (b) M. Iwaoka, S. Takamoto and S. Tomoda, J. Am. Chem. Soc., 2002, 124, 10613 CrossRef CAS PubMed.
- Y. Nagao, T. Hirata, S. Goto, S. Sano, A. Kakehi, K. Iizuka and M. Shiro, J. Am. Chem. Soc., 1998, 120, 3104 CrossRef CAS.
- (a) J. S. Murray, P. Lane, T. Clark and P. Politzer, J. Mol. Model., 2007, 13, 1033 CrossRef CAS PubMed; (b) S. Tsuzuki and N. Sato, J. Phys. Chem. B, 2013, 117, 6849 CrossRef CAS PubMed.
- (a) T. Clark, M. Hennemann, J. S. Murray and P. Politzer, J. Mol. Model., 2007, 13, 291 CrossRef CAS PubMed; (b) P. Politzer and J. S. Murray, ChemPhysChem, 2013, 14, 278 CrossRef CAS PubMed.
- (a) G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed; (b) R. Wilcken, M. O. Zimmermann, A. Lange, A. C. Joerger and F. M. Boeckler, J. Med. Chem., 2013, 56, 1363 CrossRef CAS PubMed.
- (a) K. E. Riley, J. S. Murray, J. Fanfrlík, J. Řezáč, R. J. Sola, M. C. Concha, F. M. Ramos and P. Politzer, J. Mol. Model., 2011, 17, 3309 CrossRef CAS PubMed; (b) M. Kolář, J. Hostaš and P. Hobza, Phys. Chem. Chem. Phys., 2014, 16, 9987 RSC; (c) M. Kolář, J. Hostaš and P. Hobza, Phys. Chem. Chem. Phys., 2015, 17, 23279 RSC.
- (a) L. A. Herdegger, B. Kuhn, B. Spinnler, L. Anselm, R. Ecabert, M. Stihle, B. Gsell, R. Thoma, J. Diez, J. Benz, J. M. Plancher, G. Hartmann, D. W. Banner, W. Haap and F. Diederich, Angew. Chem., Int. Ed., 2011, 50, 314 CrossRef PubMed; (b) J. Fanfrlík, M. Kolář, M. Kamlar, D. Hurny, F. X. Ruiz, A. Cousido-Siah, A. Mitschler, J. Řezáč, E. Munusamy, M. Lepšík, P. Matejicek, J. Vesely, A. Podjarny and P. Hobza, ACS Chem. Biol., 2013, 8, 2484 CrossRef PubMed.
- J. Fanfrlík, A. Přáda, Z. Padělková, A. Pecina, J. Macháček, M. Lepšík, J. Holub, A. Růžička, D. Hnyk and P. Hobza, Angew. Chem., Int. Ed., 2014, 53, 10139 CrossRef PubMed.
- W. Wang, B. Ji and Y. Zhang, J. Phys. Chem. A, 2009, 113, 8132 CrossRef CAS PubMed.
- (a) V. Nziko and S. Scheiner, J. Phys. Chem. A, 2014, 118, 10849 CrossRef CAS PubMed; (b) For the molecular structures of molecules with two- and four-coordinated sulfsur see I. Hargittai, The Structure of Volatile Sulphur Compounds, Akadémiai Kiadó, Budapest, 1985 Search PubMed.
- A. Pecina, M. Lepsik, D. Hnyk, P. Hobza and J. Fanfrlík, J. Phys. Chem. A, 2015, 119, 1388 CrossRef CAS PubMed.
- A. Bauza, D. Quinonero, P. M. Deya and A. Frontera, CrystEngComm, 2013, 15, 3137 RSC.
- W. Zierkiewicz, J. Fanfrlík, P. Hobza, D. Michalska and T. Zeegers-Huyskens, Theor. Chem. Acc., 2016, 135, 217 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- (a) P. Flűkiger, H. P. Lűthi, S. Portmann and J. Weber, MOLEKEL 4.3, Swiss Center for Scientific Computing, Manno Switzerland, 2000 Search PubMed; (b) S. Portmann and H. P. Lűthi, MOLEKEL: CHIMIA, 2007, vol. 28, p. 555 Search PubMed; (c) F. A. Bulat, A. Toro-Labbe, T. E. Brinck, J. S. Murray and P. Politzer, J. Mol. Model., 2010, 16, 1679 CrossRef CAS PubMed; (d) K. E. Riley, K.-A. Tran, P. Lane, J. S. Murray and P. Politzer, J. Comput. Sci. DOI:10.1016/j.jocs.2016.03.010; (e) C. I. Bayly, P. Cieplak, W. D. Cornell and P. A. Kollman, J. Phys. Chem., 1993, 97, 10269 CrossRef CAS.
- Borane architecture is based on three-dimensional structures composed of triangular B-B-B and B-H-B units, the latter representing a hydrogen bridge. The unique architectures of binary boron hydrides (stable as dianions, BnHn2−) stem from the number of boron vertices n (in the basic series n = 5–12) and electrons available – we thus distinguish closed-cage closo-species (2n + 2 electrons) and several open-cage species, derived by the formal removal one (nido-, 2n + 4 electrons), two (arachno-, 2n + 6 electrons) or more (hypho-, 2n + 8 electrons) boron vertices. See e.g. D. Hnyk and D. A. Wann, Boron – the Fifth Element, Challenges and Advances in Computational Chemistry and Physics, ed. D. Hnyk and M. McKee, Springer, Heidelberg, New York, Dordrecht and London, 2015, ch. 2, vol. 20 Search PubMed.
- (a) All such properties and subsequent applications are reviewed in J. Plešek, Chem. Rev., 1992, 92, 269 CrossRef; (b) Boron Science – New Technologies and Applications, ed. N. S. Hosmane, CRC Press, Boca Raton, 2012 Search PubMed.
- R. Sedlak, J. Fanfrlík, A. Pecina, D. Hnyk, P. Hobza and M. Lepšík, Boron – the Fifth Element, Challenges and Advances in Computational Chemistry and Physics, ed. D. Hnyk and M. McKee, Springer, Dordrecht, The Netherlands, 2015, ch. 9, vol. 20 Search PubMed.
- J. Fanfrlík, M. Lepšík, D. Hořínek, Z. Havlas and P. Hobza, ChemPhysChem, 2006, 7, 1100 CrossRef PubMed.
- E. D. Jemmis and E. G. Jaysree, Acc. Chem. Res., 2003, 36, 816 CrossRef CAS PubMed.
- K. Hedberg and V. Schomaker, J. Am. Chem. Soc., 1951, 73, 1482 CrossRef CAS.
- W. N. Lipscomb, Boron Hydrides, Benjamin, New York, 1963 Search PubMed.
- D. Hnyk, V. Všetečka, L. Drož and O. Exner, Collect. Czech. Chem. Commun., 2001, 66, 1375 CrossRef CAS.
- J. Fanfrlík, D. Hnyk, M. Lepšík and P. Hobza, Phys. Chem. Chem. Phys., 2007, 9, 2085 RSC.
- The presence of oxygen in a single-cluster borane framework has been achieved once in nido-[OB11H12]−, see A. Ouassas, B. Fenet, H. Mongeot, B. Gautheron, E. Bardky and B. Frange, J. Chem. Soc., Chem. Commun., 1995, 1663 RSC.
- The gas-phase electron diffraction structure together with computations of 11B NMR is reported in D. Hnyk, E. Vajda, M. Bühl and P. v. R. Schleyer, Inorg. Chem., 1992, 31, 2464 CrossRef CAS.
- D. Hnyk, D. A. Wann, J. Holub, M. Bühl, H. E. Robertson and D. W. H. Rankin, Dalton Trans., 2008, 96 RSC.
- Not only the electron diffraction structure but also the mechanism of the antipodal effect is reported in D. Hnyk, D. A. Wann, J. Holub, S. Samdal and D. W. H. Rankin, Dalton Trans., 2011, 40, 5734 RSC . The antipodal effect is based on the occurrence of paramagnetic contributions to the shielding tensor. These contributions arise from the coupling of HOMO and LUMO orbitals that have predominant coefficients on the Y and antipodal atoms. 11B chemical shift were computed using Gaussian 09, ref. 15.
- The insertion of Te into a boron cluster is mentioned in G. D. Friesen and L. J. Todd, J. Chem. Soc., Chem. Commun., 1978, 349 RSC.
- (a) P. v. R. Schleyer, K. Najafian and A. M. Mebel, Inorg. Chem., 1998, 37, 6765 CrossRef CAS PubMed; (b) A. P. M. Robertson, N. A. Beattie, G. Scott, W. Y. Man, J. J. Jones, S. A. Macgregor, G. M. Rosair and A. J. Welch, Angew. Chem., Int. Ed., 2016, 55, 8706 CrossRef CAS PubMed; (c) P. v. R. Schleyer and K. Najafian, Inorg. Chem., 1998, 37, 3454 CrossRef CAS PubMed.
- A. J. Stone and M. J. Alderton, Inorg. Chem., 1982, 21, 2297 CrossRef CAS , and the references therein.
- T. Baše, Z. Bastl, M. Šlouf, M. Klementová, J. Šubrt, A. Vetushka, M. Ledinský, A. Fejfar, J. Macháček, M. J. Carr and M. G. S. Londesborough, J. Phys. Chem. C, 2008, 112, 14446 Search PubMed.
- D. A. Wann, P. D. Lane, H. E. Roberson, T. Baše and D. Hnyk, Dalton Trans., 2013, 42, 12015 RSC.
- This compound has been recently prepared and structurally characterized in terms of electron diffraction and NMR spectroscopy.
- This compound has not been synthesized yet.
- R. Lo, J. Fanfrlik, M. Lepsik and P. Hobza, Phys. Chem. Chem. Phys., 2015, 17, 20814 RSC.
- D. Hnyk, V. Všetečka and L. Drož, J. Mol. Struct., 2010, 978, 246 CrossRef CAS.
- M. Lepšík, M. Srnec, J. Plešek, M. Buděšínský, B. Klepetářová, D. Hnyk, B. Grüner and L. Rulíšek, Inorg. Chem., 2010, 49, 5040 CrossRef PubMed.
- Not only closo systems but also neutral nido clusters are potential candidates for the examination of σ-hole interactions; for some neutral nido-heteroboranes, see e.g. (a) D. Hnyk, M. Hofmann, P. v. R. Schleyer, M. Bühl and D. W. H. Rankin, J. Phys. Chem., 1996, 100, 3435 CrossRef CAS; (b) B. Štíbr, O. L. Tok, J. Holub, M. Bakardjiev, W. Milius, D. Hnyk and B. Wrackmeyer, Eur. J. Inorg. Chem., 2002, 2320 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |