Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An autonomic self-healing organogel with a photo-mediated modulus†
Yubing
Xiong
ab,
Zhijun
Chen
a,
Hong
Wang
c,
Lisa-Maria
Ackermann
a,
Markus
Klapper
a,
Hans-Jürgen
Butt
a and
Si
Wu
*a
aMax Planck Institute for Polymer Research, Ackermannweg 10, Mainz 55128, Germany. E-mail: wusi@mpip-mainz.mpg.de
bKey Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education; College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, China
cPhysical Science and Engineering Division, King Abdullah University of Science & Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia
First published on 15th November 2016
Abstract
A new method is described for fabricating autonomic, self-healing, deformable organogels. We combined imidazolium-based poly(ionic liquid) (PIL) and azobenzene-grafted poly(carboxylic acid) (PAA-Azo) in N,N-dimethyl formamide. Further, complexing PIL with unirradiated (trans) or irradiated (cis) PAA-Azo tuned the elastic modulus of the organogel.
Polymeric networks mediated by non-covalent interactions are more sensitive to their external environment and return more easily to their original state compared to covalently-cross-linked networks.1 As a result, they have attracted significant attention in materials and chemical science.2 Owing to the unique features of the weak interactions, these polymer networks can respond to external stimuli while maintaining stability and functionality similar to covalently bonded systems.3 Although significant progress has been made in organogels, exploring novel materials with intrinsic self-healing properties via non-covalent interactions,4 such as the ubiquitous cation–π interaction, is of outstanding importance in determining the structure and function of supramolecular assemblies in chemistry, materials science, and biology.5
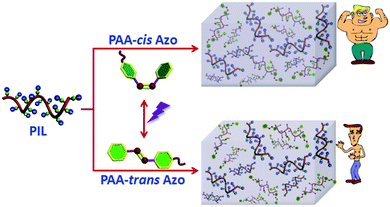
Poly(ionic liquid)s (PILs), derived from the polymerization of ionic liquid (IL)-based monomers, can combine some unique features of ILs with the mechanical properties of polymers.6 Thus, PILs are of interest for solid ion conductors, CO2 conversion, porous materials, and carbon precursors.7 However, there are few reports on the fabrication of PIL-based supramolecular gels through non-covalent interactions. Herein, we report a novel supramolecular organogel which is prepared by combining imidazolium (Im)-based PIL, poly(3-cyanomethyl-1-vinyl imidazolium bis(trifluoromethane sulfonyl)imide) (PCMVImTf2N), and azobenzene (Azo)-grafted poly(acrylic acid) (PAA-Azo). PIL@PAA-Azo organogels are both self-healable and stretchable (Scheme 1). Yet, they shrink in the presence of guanidine hydrochloride (Gdn-HCl). Additionally, azobenzene groups show reversible cis–trans photoisomerization and PIL@PAA-Azo organogels have a higher modulus when PIL is complexed with PAA-cis-Azo than with PAA-trans-Azo. These versatile properties of PIL@PAA-Azo organogels were understood in terms of the synergistic interactions of cation–π and H-bonding.
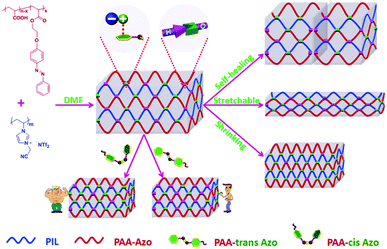 | ||
| Scheme 1 Schematic illustration of preparation of a PIL@PAA-Azo organogel and its versatile performances. | ||
PCMVImTf2N with a molecular weight of 1.12 × 106 g mol−1 was prepared by free radical polymerization (Scheme S1, Fig. S1 and S2, ESI†). PAA-Azo was prepared by grafting azobenzene groups onto PAA (MW = 2.5 × 105 g mol−1) (Scheme S2, ESI†).8 The grafting density measured using 1H NMR spectroscopy was 11.3 mol%. PIL@PAA-Azo organogels were prepared by mixing DMF solutions of PCMVImTf2N and PAA-Azo. Although the solvent for both polymer solutions was DMF, the two polymer solutions initially separated after combining the PAA-Azo and PIL solutions (Fig. S3, ESI†). After agitating and standing for several minutes, a semitransparent organogel was obtained, which was confirmed via vial inversion. When the mass ratio of PIL to PAA-Azo was 4 to 1, only a high-viscosity solution was obtained rather than an organogel (Fig. 1). However, all the solutions were gelated when the mass ratio was less than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For comparison, PIL solution was also mixed with pure PAA under identical conditions (PIL
1. For comparison, PIL solution was also mixed with pure PAA under identical conditions (PIL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAA = 2
PAA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) and no gelation occurred. These results confirmed that the introduction of azobenzene groups was critical for the formation of the organogels.
1, w/w) and no gelation occurred. These results confirmed that the introduction of azobenzene groups was critical for the formation of the organogels.
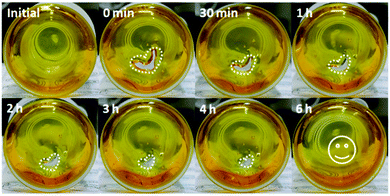
PIL@PAA-Azo organogels exhibit some interesting features. Firstly, the organogel could autonomically self-heal after it was damaged. When two pieces of gel were kept in contact with each other without applying pressure (Video 1, ESI†), the two gels joined together quickly. The combined pieces did not separate even by pulling from two different ends. To demonstrate the self-healing ability of PIL@PAA-Azo organogels more practically, a PIL@PAA-Azo organogel was prepared in a vial and cut with a notch (Fig. 2). Without any external stimuli, the notch became smaller and disappeared completely within 6 h. These results demonstrated that the PIL@PAA-Azo organogel can self-heal autonomically after damage. Additionally, the PIL@PAA-Azo organogel is highly stretchable (Video 2, ESI†). The organogel can withstand the tensile stress of elongation to 5 times its original length.
A PIL@PAA-Azo organogel with a PIL to PAA-Azo ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) was studied using dynamic rheology (Fig. S4, ESI†). PIL@PAA-Azo presented a higher storage modulus (G′) than loss modulus (G′′) within the frequency range investigated. This indicated quasi-solid-state behavior, confirming the formation of an organogel. Trans–cis isomerization of Azo has been widely utilized to fabricate gel–sol transitions and association/dissociation systems.10 To that end, the photoresponse of PIL@PAA-Azo organogels was studied using UV-vis spectroscopy. As depicted in Fig. S5 (ESI†), PAA-Azo exhibited π–π* and n–π* transition absorption bands at 352 nm and 446 nm, respectively. Approximately 65% trans-isomer in PAA-Azo transformed into a cis-isomer under UV irradiation (0.45 mW cm−2) for 90 s. After subsequent green light irradiation (0.9 mW cm−2) for 90 s, the cis-isomer completely transformed back into the trans-isomer. These results demonstrate that the reversible isomerization of PAA-Azo can be mediated by alternative UV and visible light irradiation. The photocontrol of the PIL@PAA-Azo organogel can alternatively be applied in solution, before the formation of the organogel. By irradiating the PAA-Azo solution with UV light before the complexation, complete isomerization of Azo units was achieved. Then, the PAA-cis-Azo solution was immediately combined with PIL and the resultant organogel was studied using dynamic rheology. Both G′ and G′′ of the PIL@PAA-cis-Azo organogel were much higher than those of the PIL@PAA-trans-Azo organogel (prepared using PAA-Azo without UV light, Fig. 3). This performance indicates that the interaction of PIL with PAA-cis-Azo was stronger than that with PAA-trans-Azo. Thus, photo-control of the bulk modulus of the PIL@PAA-Azo organogel was achieved. The mechanism of the photo-controlled modulus is presented below.
1 (w/w) was studied using dynamic rheology (Fig. S4, ESI†). PIL@PAA-Azo presented a higher storage modulus (G′) than loss modulus (G′′) within the frequency range investigated. This indicated quasi-solid-state behavior, confirming the formation of an organogel. Trans–cis isomerization of Azo has been widely utilized to fabricate gel–sol transitions and association/dissociation systems.10 To that end, the photoresponse of PIL@PAA-Azo organogels was studied using UV-vis spectroscopy. As depicted in Fig. S5 (ESI†), PAA-Azo exhibited π–π* and n–π* transition absorption bands at 352 nm and 446 nm, respectively. Approximately 65% trans-isomer in PAA-Azo transformed into a cis-isomer under UV irradiation (0.45 mW cm−2) for 90 s. After subsequent green light irradiation (0.9 mW cm−2) for 90 s, the cis-isomer completely transformed back into the trans-isomer. These results demonstrate that the reversible isomerization of PAA-Azo can be mediated by alternative UV and visible light irradiation. The photocontrol of the PIL@PAA-Azo organogel can alternatively be applied in solution, before the formation of the organogel. By irradiating the PAA-Azo solution with UV light before the complexation, complete isomerization of Azo units was achieved. Then, the PAA-cis-Azo solution was immediately combined with PIL and the resultant organogel was studied using dynamic rheology. Both G′ and G′′ of the PIL@PAA-cis-Azo organogel were much higher than those of the PIL@PAA-trans-Azo organogel (prepared using PAA-Azo without UV light, Fig. 3). This performance indicates that the interaction of PIL with PAA-cis-Azo was stronger than that with PAA-trans-Azo. Thus, photo-control of the bulk modulus of the PIL@PAA-Azo organogel was achieved. The mechanism of the photo-controlled modulus is presented below.
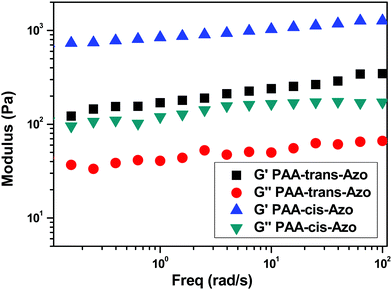 | ||
Fig. 3 Rheological properties of the 10 wt% PIL@PAA-Azo organogel (PIL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAA-Azo = 1 PAA-Azo = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) at 25 °C (storage modulus G′ and loss modulus G′′ as a function of frequency). 1, w/w) at 25 °C (storage modulus G′ and loss modulus G′′ as a function of frequency). | ||
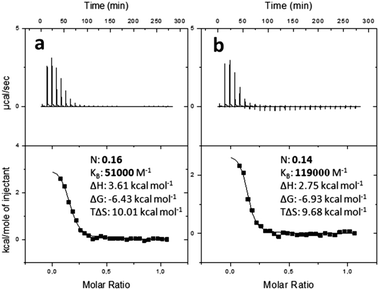
The different binding abilities of PAA-trans-Azo and PAA-cis-Azo with PIL were estimated using isothermal titration calorimetry (ITC). The enthalpy changes (ΔH) in the process of mixing PIL solution (5 mM−1) with PAA-Azo solution (1 mM−1) before and after UV irradiation were 3.61 kcal mol−1 and 2.75 kcal mol−1 (Fig. 4). Fig. 4 also illustrates that the binding affinity (KB) for PIL and PAA-Azo was 5.1 × 104 M−1 before and 1.19 × 105 M−1 after UV light irradiation. Thus, PIL was more inclined to bind PAA-cis-Azo rather than PAA-trans-Azo, consistent with the rheology results. The binding affinity of PIL with PAA-cis-Azo is also much higher than alpha cyclodextrin compounds with trans-Azo.10 As a result, a much stronger organogel can be obtained by complexing PIL with PAA-cis-Azo compared to previous work (Scheme 2). And, in addition, the modulus of the PIL@PAA-Azo organogel can be tuned through photo-induced isomerization of PAA-Azo.
Im-IL has been found to form H-bonding interactions with polar molecules.11 Thus, H-bonding between carboxyl groups and Im-IL is likely one of the driving forces for the formation of the PIL@PAA-Azo organogel. Since it is unlikely that an organogel can be produced only by H-bonds, there must be some other interactions between the Azo groups and PIL. Based on the structures of PIL and PAA-Azo, we suggest that cation–π interactions between PIL and PAA-Azo lead to the formation of a gel. To verify our hypothesis, 1H NMR spectroscopy was utilized to investigate the interactions between PIL and PAA-Azo. Curves a and b in Fig. S6 (ESI†) depict the resonance absorption signal of PAA-Azo and PIL, respectively. All the characteristic signals ascribed to azobenzene and Im units were recognized, such as trans-Azo (7.16, 7.54, 7.85 ppm), Im (9.22, 7.82 ppm) and N–CH2–CN (5.49 ppm). Additionally, the proton signal ascribed to free –COOH disappeared, which was probably due to deuterium–hydrogen exchange in the presence of trace water. However, the proton signal ascribed to –COOH emerged when some PIL was added into PAA-Azo solution. This is probably because that H-bonding between PIL and PAA-Azo can prevent the deuterium–hydrogen exchange. Moreover, a new signal (6.84 ppm) emerges after the addition of PIL. Simultaneously, the signal ascribed to the Im unit (9.22 ppm) shifted to a low field, which probably points to an interaction between Im and Azo units. When more PIL was added, the new signal also enhanced a little. Subsequently, the sample was irradiated by UV light. It can be found that the new signal enhanced greatly and the trans-Azo signal attenuated. After green light irradiation, the new signal also attenuated. The results demonstrated that the new signal is ascribed to cis-Azo, and the formation of cis-Azo is possible because of the presence of PIL.
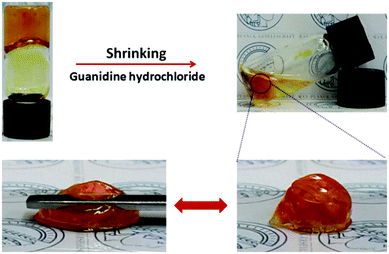
Additionally, shrinking of the PIL@PAA-Azo organogel was observed when it was treated with Gdn-HCl (Fig. 5). The treated organogel became much stronger than the one before Gdn-HCl treatment (Video 3, ESI†). Since Gdn-HCl is known to break H-bonds between biomacromolecules, this toughening likely stems from an increase in cation–π interactions (see below), which are stronger and thus toughen the gel.9 Thus H-bonding is one of the forces driving the formation of the organogel and determining its properties of deformation.
According to the above results, a plausible mechanism for the versatile performance of a PIL@PAA-Azo organogel was postulated. Cation–π and H-bonding interactions were taken as the main driving forces for the formation of the organogel. When the gels are damaged by an external force, new cation–π and H-bonding interactions can take place on the interface between two pieces of organogel. Therefore, the broken organogel can reform intermolecular bonds to self-heal. Compared with cation–π interactions, the H-bonding interaction is relatively weaker.12 Thus, H-bonds between PIL and PAA-Azo are more easily destroyed during stretching, whereas cation–π interactions more likely remain intact. However, in the presence of Gdn-HCl, most H-bonds will break while leaving the cation–π interactions unaffected. Simultaneously, due to the cleavage of H-bonds, some new binding sites for cation–π interactions will become available. Thus, the solvent is extruded due to the cleavage of the H-bonding network and formation of new cation–π interaction which causes the PIL@PAA-Azo organogel to shrink.
In summary, a novel autonomic, self-healing, and deformable organogel was prepared by complexing PIL and PAA-Azo in DMF. The formation of the PIL@PAA-Azo organogel was due to H-bonding and cation–π interactions. The modulus of the PIL@PAA-Azo organogel was photo-tuned by combining PIL with different Azo isomers. Our findings thus provide an alternative tactic for fabricating self-healing materials with a photo-tunable modulus. These organogels may be used as solid electrolytes, which can find extensive application in the fields of supercapacitors, batteries, and flexible electronics.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, WU 787/2-1) and the National Natural Science Foundation of China (NSFC 21474080). Y. X. was supported by the CSC program. Thanks go to Dr K. Koynov and A. Hanewald (MPIP) for help with the dynamic rheology measurements.
Notes and references
- S. Burattini, B. W. Greenland, D. Chappell, H. M. Colquhoun and W. Hayes, Chem. Soc. Rev., 2010, 39, 1973 RSC; M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef CAS PubMed; Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446 RSC; X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165 CrossRef PubMed; B. L. Zhu, N. Jasinski, A. Benitez, M. Noack, D. Park, A. S. Goldmann, C. Barner-Kowollik and A. Walther, Angew. Chem., Int. Ed., 2015, 54, 8653 CrossRef PubMed; X. Yan, D. Xu, J. Chen, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 3312 RSC.
- L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196 CrossRef CAS PubMed; L. Zhang, H. Liang, J. Jacob and P. Naumov, Nat. Commun., 2015, 6, 7429 CrossRef PubMed; O. Kotova, R. Daly, C. M. G. dos Santos, M. Boese, P. E. Kruger, J. J. Boland and T. Gunnlaugsson, Angew. Chem., Int. Ed., 2012, 51, 7208 CrossRef PubMed; T. Ogoshi, Y. Ichihara, T. A. Yamagishi and Y. Nakamoto, Chem. Commun., 2010, 46, 6087 RSC; J. N. Hunt, K. E. Feldman, N. A. Lynd, J. Deek, L. M. Campos, J. M. Spruell, B. M. Hernandez, E. J. Kramer and C. J. Hawker, Adv. Mater., 2011, 23, 2327 CrossRef PubMed; H. Zeng, D. S. Hwang, J. N. Israelachvili and J. H. Waite, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 12850 CrossRef PubMed.
- X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC; Y. Chen, A. M. Kushner, G. A. Williams and Z. Guan, Nat. Chem., 2012, 4, 467 CrossRef CAS PubMed; J. Cui, D. Daniel, A. Grinthal, K. Lin and J. Aizenberg, Nat. Mater., 2015, 14, 790 CrossRef PubMed; J. Cui and A. del Campo, Chem. Commun., 2012, 48, 9302 RSC; L. Li, B. Yan, J. Yang, L. Chen and H. Zeng, Adv. Mater., 2015, 27, 1294 CrossRef PubMed.
- F. Zeng, Y. Shen and C. F. Chen, Soft Matter, 2013, 9, 4875 RSC.
- J. C. Ma and D. A. Dougherty, Chem. Rev., 1997, 97, 1303 CrossRef CAS PubMed; A. S. Mahadevi and G. N. Sastry, Chem. Rev., 2013, 113, 2100 CrossRef PubMed; D. A. Dougherty, Acc. Chem. Res., 2013, 46, 885 CrossRef PubMed; Q. Lu, D. X. Oh, Y. Lee, Y. Jho, D. S. Hwang and H. Zeng, Angew. Chem., Int. Ed., 2013, 52, 3944 CrossRef PubMed.
- D. Mecerreyes, Prog. Polym. Sci., 2011, 36, 1629 CrossRef CAS; J. Yuan, D. Mecerreyes and M. Antonietti, Prog. Polym. Sci., 2013, 38, 1009 CrossRef.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Polym. Chem., 2015, 6, 2163 RSC; Y. Xiong, H. Wang, R. Wang, Y. Yan, B. Zheng and Y. Wang, Chem. Commun., 2010, 46, 3399 RSC; P. Zhang, J. Yuan, T. P. Fellinger, M. Antonietti, H. Li and Y. Wang, Angew. Chem., Int. Ed., 2013, 52, 6028 CrossRef CAS PubMed; J. Steinkoenig, F. R. Bloesser, B. Huber, A. Welle, V. Trouillet, S. M. Weidner, L. Barner, P. W. Rroesky, J. Y. Yuan, A. S. Goldmann and C. Barner-Kowllik, Polym. Chem., 2016, 7, 451 RSC; Q. Zhao, P. Zhang, M. Antonietti and J. Yuan, J. Am. Chem. Soc., 2012, 134, 11852 CrossRef PubMed; Q. Zhao, J. Heyda, J. Dzubiella, K. Täuber, J. W. C. Dunlop and J. Yuan, Adv. Mater., 2015, 27, 2913 CrossRef PubMed; Q. Zhao, S. Soll, M. Antonietti and J. Yuan, Polym. Chem., 2013, 4, 2432 RSC.
- D. S. Wang, M. Wagner, H. J. Butt and S. Wu, Soft Matter, 2015, 11, 7656 RSC; S. Wu, Q. Zhang and C. Bubeck, Macromolecules, 2010, 43, 6142 CrossRef CAS; D. Wang and S. Wu, Langmuir, 2016, 32, 632 CrossRef PubMed.
- S. Cui, C. Albrecht, F. Kühner and H. E. Gaub, J. Am. Chem. Soc., 2006, 128, 6636 CrossRef CAS PubMed.
- X. Liao, G. Chen, X. Liu, W. Chen, F. Chen and M. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4409 CrossRef CAS PubMed; S. Tamesue, Y. Takashima, H. Yamaguchi, S. Shinkai and A. Harada, Angew. Chem., Int. Ed., 2010, 49, 7461 CrossRef PubMed.
- Y. Xiong, J. Liu, Y. Wang, H. Wang and R. Wang, Angew. Chem., Int. Ed., 2012, 51, 9114 CrossRef CAS PubMed.
- S. Y. Jon, J. Kim, M. Kim, S. H. Park, W. S. Jeon, J. Heo and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 2116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc08513j |
| This journal is © The Royal Society of Chemistry 2016 |