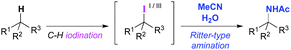Open Access Article
Open Access ArticleRitter-type amination of C–H bonds at tertiary carbon centers using iodic acid as an oxidant†
Kensuke
Kiyokawa
*,
Kenta
Takemoto
and
Satoshi
Minakata
*
Department of Applied Chemistry, Graduate School of Engineering Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan. E-mail: kiyokawa@chem.eng.osaka-u.ac.jp; minakata@chem.eng.osaka-u.ac.jp
First published on 22nd September 2016
Abstract
The Ritter-type amination of a tertiary C–H bond using iodic acid (HIO3) as an oxidant, in the presence of N-hydroxyphthalimide (NHPI) is reported. This operationally simple method is conducted under metal-free conditions, is scalable, and efficiently provides valuable α-tertiary amine derivatives.
Incorporation of an amino functional group into an organic molecule via the direct conversion of a C(sp3)–H bond to a C(sp3)–N bond is one of the most powerful and straightforward strategies for preparing nitrogen-containing organic compounds.1 The intermolecular, direct amination of C–H bonds at tertiary carbon centers is a particularly attractive transformation that produces α-tertiary amine derivatives, which are potentially useful building blocks for new types of drugs and in medicinal chemistry.2 In the past decades, the insertion of a nitrene has evolved to be one of the most reliable methods for the direct amination of C(sp3)–H bonds. However, in the case of reactions that involve unactivated tertiary C–H bonds, a superstoichiometric amount of substrate is required. As a result, its use in functionalizing valuable complex molecules has remained undeveloped.3,4 To overcome these problem, Du Bois et al. recently reported on an elegant method for selective tertiary C–H amination using the originally developed rhodium catalyst.5 In this reaction, the substrate can be used as the limiting reagent. In addition, most recently, tertiary C–H azidation has also emerged as a promising approach,6 and these methods were reported to be applicable to the late stage functionalization of complex molecules. However, despite these advances, the direct and site-selective conversion of an unactivated tertiary C–H bond to a C–N bond, particularly under metal-free conditions, continues to be a challenging and highly demanding task.7,8
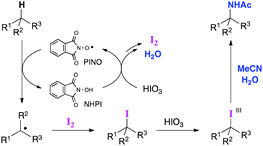
Ritter-type C–H aminations, where nitriles function as the nitrogen source, are a potentially efficient strategy for intermolecular tertiary C–H amination reactions. However, only a few oxidation systems have been reported that are suitable for the reaction, and thus its applications have been quite limited to date. Early reports were limited to the reaction of adamantane derivatives.9 Although the amination of benzylic C–H bonds using N-hydroxyphthalimide (NHPI) combined with ceric ammonium nitrate (CAN) as an oxidant was developed by Ishii et al., the reaction with respect to tertiary C–H bonds, with the exception of adamantane derivatives, has not been extensively explored.10 Baran et al. recently developed a practical Ritter-type tertiary C–H amination involving a guided C–H oxidation approach using substoichiometric amounts of copper and zinc salts.11 The proposed reaction pathway involves the metal-oxidant-mediated single-electron oxidation of an in situ generated alkyl radical species to produce a carbocation intermediate. To develop a more efficient Ritter-type tertiary C–H amination that does not involve the use of metal oxidants, we conceived a new approach using iodine-based oxidants, in which C–H iodination would be induced to provide tertiary alkyl iodides and the corresponding iodine(III) species with a higher oxidation state that could undergo a subsequent substitution by nitriles (Scheme 1).12 Herein, we report on the Ritter-type amination of unactivated tertiary C–H bonds using iodic acid (HIO3) as an oxidant in the presence of NHPI.
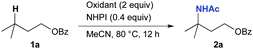
We began our investigation by screening oxidants for the amination of isoamyl benzoate (1a), which is used as a limiting reagent (Table 1).13 On the basis of the reaction conditions reported by Ishii,10 we first surveyed the effect of several common oxidants for the reaction in the presence of NHPI in MeCN at 80 °C for 12 h. When CAN was used, tertiary C–H amination proceeded to give 2a in low yield (entry 1).
| Entry | Oxidant | Yieldb [%] |
|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), oxidant (0.4 mmol), NHPI (0.08 mmol), MeCN (1 mL), 80 °C. b Determined by 1H NMR analysis of the crude product using 1,1,2,2-tetrachloroethane as an internal standard. Yield in parenthesis is the isolated yield based on the amount of 1a used. c I2 (0.5 equiv.) was added. d Reactions were conducted on a 0.4 mmol scale. e Reactions were conducted using HIO3 (0.8 equiv.), NHPI (0.2 equiv.), and MeCN (2 mL) for 24 h. f Reaction was conducted without NHPI. g Reaction was conducted under dark. | ||
| 1 | CAN | 19 |
| 2 | Oxone | 0 |
| 3 | mCPBA | 0 |
| 4 | PhI(OAc)2 | <5 |
| 5c | CAN | 53 |
| 6c | Oxone | 69 |
| 7c | mCPBA | 65 |
| 8c | PhI(OAc)2 | <5 |
| 9d | HIO3 | 72 |
| 10d,e | HIO3 | 74 (71) |
| 11d,e | none | 0 |
| 12d,e,f | HIO3 | 0 |
| 13d,e,g | HIO3 | 72 |
Neither Oxone nor m-chloroperbenzoic acid (mCPBA) showed any reactivity for this reaction (entries 2 and 3), and PhI(OAc)2 gave only a trace amount of 2a (entry 4). Next, to trap the in situ generated alkyl radical species, we examined the reaction using CAN with the addition of 0.5 equiv. of I2, as an iodine source, and were pleased to find that the amination proceeded efficiently, affording 2a in 53% yield (entry 5). Interestingly, when I2 was present in the reaction system, both Oxone and mCPBA also effectively promoted the amination (entries 6 and 7) although they were completely inactive when used as the sole oxidant. Meanwhile, 2a was not produced when PhI(OAc)2 was used in the reaction (entry 8). These results prompted us to explore a more effective oxidant for this reaction. We hypothesized that iodic acid (HIO3) and iodine pentoxide (I2O5) might be promising oxidants14 since they are commercially available, low cost, and stable even at high temperatures and can be reduced in situ to generate I2 and H2O,14k,l both of which are requisite components in this Ritter-type amination. When we examined the use of HIO3, the amination proceeded efficiently without the need for added I2, affording 2a in 72% yield (entry 9).15 Furthermore, attempts to reduce the amount of reagents employed revealed that the use of HIO3 (0.8 equiv.) and NHPI (0.2 equiv.) in conjunction with a longer reaction time of 24 h provided 2a in 74% yield (entry 10).13 Control experiments in the absence of either HIO3 or NHPI revealed that both reagents were required for this transformation (entries 11 and 12). In addition, the reaction does not require a light-induced process since the reaction proceeded equally well, even in the dark (entry 13).
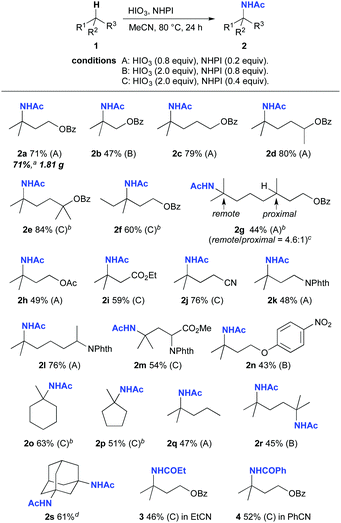
We next investigated the ability and scope of this Ritter-type C–H amination (Scheme 2). To establish the synthetic utility of this amination, we carried out the reaction using 1a on a gram-scale (10 mmol), which resulted in the formation of 2a in 71% yield (1.81 g).13 Electronic effects on the reaction were evaluated by using a series of substrates bearing a benzoate group as an electron-withdrawing group (EWG). Isobutyl benzoate, which contains a tertiary C–H moiety β to the oxygen atom of the benzoate group, showed a low reactivity because of the electron deficient character of the C–H bond, and the product 2b was obtained in a relatively lower yield than that for 2a. In contrast, a tertiary C–H bond remote from the benzoate group showed a high reactivity (2c). Similarly, secondary and tertiary alcohol derivatives as well as primary alcohols were also suitable for use in this reaction system, providing 2d and 2e in high yields, respectively. The replacement of a methyl group with an ethyl group at the reaction site had a slight effect on the reaction (2f). When a benzoate derived from 3,7-dimethyl-1-octanol, which has two tertiary carbon centers, was examined, the tertiary C–H bond remote from the benzoate group selectively underwent amination over the proximal site, selectively furnishing 2g (remote/proximal = 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).13 This selectivity can be attributed to the electronic effect by an EWG, and this trend is consistent with previously reported findings on selectivity for the oxidation of C–H bonds.6b,16 Substrates bearing functional groups, including an acetoxy protected alcohol (2h), an ester (2i), a nitrile (2j), phthaloyl protected amines (2k and 2l), and a leucine derivative (2m), were also compatible with the reaction. The amination of a substrate containing a phenoxy moiety, leading to a medicinally important phenoxypropylamine framework, was sluggish because of the competitive electrophilic iodination of the aromatic ring. Therefore, to prevent this side reaction, the effect of the incorporation of a nitro group at the para position in the phenyl ring was examined, which resulted in the formation of the desired 2n. Furthermore, in this system, cyclic and noncyclic hydrocarbons also underwent selective C–H amination at tertiary carbon centers (2o–2s). It is noteworthy that a double C–H amination was successfully achieved in the reactions of 2,5-dimethylhexane as well as adamantane (2r and 2s).13 Finally, the use of propionitrile and benzonitrile as a solvent instead of acetonitrile provided the corresponding products 3 and 4, respectively.
1).13 This selectivity can be attributed to the electronic effect by an EWG, and this trend is consistent with previously reported findings on selectivity for the oxidation of C–H bonds.6b,16 Substrates bearing functional groups, including an acetoxy protected alcohol (2h), an ester (2i), a nitrile (2j), phthaloyl protected amines (2k and 2l), and a leucine derivative (2m), were also compatible with the reaction. The amination of a substrate containing a phenoxy moiety, leading to a medicinally important phenoxypropylamine framework, was sluggish because of the competitive electrophilic iodination of the aromatic ring. Therefore, to prevent this side reaction, the effect of the incorporation of a nitro group at the para position in the phenyl ring was examined, which resulted in the formation of the desired 2n. Furthermore, in this system, cyclic and noncyclic hydrocarbons also underwent selective C–H amination at tertiary carbon centers (2o–2s). It is noteworthy that a double C–H amination was successfully achieved in the reactions of 2,5-dimethylhexane as well as adamantane (2r and 2s).13 Finally, the use of propionitrile and benzonitrile as a solvent instead of acetonitrile provided the corresponding products 3 and 4, respectively.
Acid hydrolysis of 2a effectively removed the acetamide moiety as well as the benzoate moiety to give 1,3-aminoalcohol 5 as the HCl salt (Scheme 3).
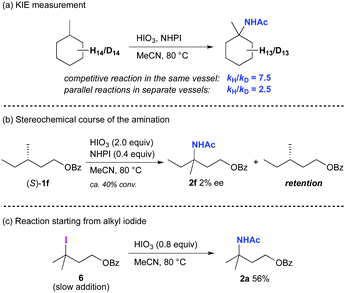
We performed several experiments in attempts to gain insights into the reaction mechanism (Scheme 4). Initially, kinetic isotope effects (KIEs) were measured with methylcyclohexane and methylcyclohexane-d14 for both competitive reactions in the same vessel and parallel reactions in separate vessels (Scheme 4a). A significant primary KIE (7.5) was observed for the competitive reaction in the same vessel, and a relatively smaller KIE (2.5) was detected when parallel reactions were carried out in separate vessels. The KIE (2.5) value in the parallel reactions indicates that C–H bond cleavage is likely the rate-determining step. In addition, C–H bond cleavage via hydrogen abstraction by a phthalimide-N-oxyl (PINO) radical was indicated by the quite large KIE (7.5) for the reaction in the same vessel.17 The difference in the KIE values between these two methods suggests that the rate of oxidation of NHPI to PINO, prior to the rate-determining C–H bond cleavage step, could affect the overall reaction rate. Next, to evaluate the stereochemical course for the reaction, the reaction using an enantiopure (S)-1f was carried out under the standard conditions (ca. 40% conversion after 6 h), resulting in the formation of nearly racemic 2f (Scheme 4b). Furthermore, the starting material was recovered with complete retention of its stereochemistry, indicating that the C–H bond cleavage is irreversible.18 These results are consistent with a reaction pathway that involves an alkyl free-radical intermediate that causes the loss of stereochemical information.7b Although we considered the possibility that an alkyl iodide could be generated in situ as a reaction intermediate, the corresponding iodine(I/III) species was not observed in the reaction system. Therefore, to get insights into the nature of the reaction intermediate, several reactions were performed employing the separately prepared alkyl iodide 6 to determine whether it is converted into 2a. Stirring a solution of 6 in MeCN at 80 °C failed to give 2a,19 while the amination proceeded in the presence of HIO3 under otherwise the same conditions to provide 2a even in low yield (9%).13 In the C–H amination under the standard conditions, an alkyl iodide could be generated in situ catalytically. With this consideration in mind, the slow addition of 6 to a suspension of HIO3 in MeCN at 80 °C was examined, and the yield of 2a was increased to 56% (Scheme 4c). These results strongly support a reaction pathway in which an alkyl iodide is generated and oxidized by HIO3 to the corresponding hypervalent iodine species, which then undergoes a Ritter-type amination.20,21
Based on the experimental results presented herein and previous reports,17 a proposed reaction pathway is depicted in Scheme 5. First, NHPI is oxidized to PINO by HIO3, which leads to the generation of I2 and H2O. Then, the rate determining C–H bond cleavage by PINO provides an alkyl radical species, which is rapidly trapped by I2. The resulting alkyl iodide is then oxidized by HIO3 to generate an iodine(III) species that undergoes a Ritter-type amination, thus affording the amide product.
In conclusion, we report on the development of a new class of metal-free Ritter-type C–H amination reactions at tertiary carbon centers using commercially available and easily handled HIO3 and NHPI. Various α-tertiary amine derivatives can be prepared by this operationally simple and environmentally benign method. Preliminary mechanistic investigations suggest that the reaction proceeds via a unique reaction pathway that involves the formation of alkyl iodides(I/III) as reaction intermediates. The present study is an important example of expanding the utility of HIO3 in organic synthesis. Further investigations of the mechanism and applications of this method to the synthesis of more complex molecules are currently underway.
This work was supported by JSPS KAKENHI Grant Number JP16K17868.
Notes and references
- (a) M. M. Díaz-Requejo and P. J. Pérez, Chem. Rev., 2008, 108, 3379 CrossRef PubMed; (b) F. Collet, R. H. Dodd and P. Dauban, Chem. Commun., 2009, 5061 RSC; (c) D. N. Zalatan and J. Du Bois, Top. Curr. Chem., 2010, 292, 347 CrossRef CAS PubMed; (d) J. Du Bois, Org. Process Res. Dev., 2011, 15, 758 CrossRef PubMed; (e) F. Collet, C. Lescot and P. Dauban, Chem. Soc. Rev., 2011, 40, 1926 RSC; (f) J. L. Roizen, M. E. Harvey and J. Du Bois, Acc. Chem. Res., 2012, 45, 911 CrossRef CAS PubMed; (g) R. T. Gephart III and T. H. Warren, Organometallics, 2012, 31, 7728 CrossRef; (h) J. L. Jeffrey and R. Sarpong, Chem. Sci., 2013, 4, 4092 RSC; (i) S. Chiba and H. Chen, Org. Biomol. Chem., 2014, 12, 4051 RSC.
- For selected reviews, see: (a) J. Clayden, M. Donnard, J. Lefranc and D. J. Tetlow, Chem. Commun., 2011, 47, 4624 RSC; (b) F. Zhou, F.-M. Liao, J.-S. Yu and J. Zhou, Synthesis, 2014, 2983 CrossRef CAS; (c) A. Hager, N. Vrielink, D. Hager, J. Lefranc and D. Trauner, Nat. Prod. Rep., 2016, 33, 491 RSC.
- For selected examples of reactions using a metal nitrenoid, see: (a) I. Nägeli, C. Baud, G. Bernardinelli, Y. Jacquier, M. Moraon and P. Müllet, Helv. Chim. Acta, 1997, 80, 1087 CrossRef; (b) K. Huard and H. Lebel, Chem. – Eur. J., 2008, 14, 6222 CrossRef CAS PubMed; (c) C. Lescot, B. Darses, F. Collet, P. Retailleau and P. Daban, J. Org. Chem., 2012, 77, 7232 CrossRef CAS PubMed.
- For metal-free C–H amination using imino-λ3-bromanes, see: (a) M. Ochiai, K. Miyamoto, T. Kaneaki, S. Hayashi and W. Nakanishi, Science, 2011, 332, 448 CrossRef CAS PubMed; (b) K. Miyamoto, T. Ota, M. M. Hoque and M. Ochiai, Org. Biomol. Chem., 2015, 13, 2129 RSC.
- J. L. Roizen, D. N. Zalatan and J. Du Bois, Angew. Chem., Int. Ed., 2013, 52, 11343 CrossRef CAS PubMed.
- (a) V. V. Zhdankin, A. P. Krasutsky, C. J. Kuehl, A. J. Simonsen, J. K. Woodward, B. Mismash and J. T. Bolz, J. Am. Chem. Soc., 1996, 118, 5192 CrossRef CAS; (b) A. Sharma and J. F. Hartwig, Nature, 2015, 517, 600 CrossRef CAS PubMed; (c) X. Huang, T. M. Bergsten and J. T. Groves, J. Am. Chem. Soc., 2015, 137, 5300 CrossRef CAS PubMed; (d) Y. Wang, G.-X. Li, G. Yang, G. He and G. Chen, Chem. Sci., 2016, 7, 2679 RSC; (e) X. Zhang, H. Yang and P. Tang, Org. Lett., 2015, 17, 5828 CrossRef CAS PubMed.
- Our group and others reported on radical aminations of tertiary C−H bonds under metal-free conditions, in which an excess amount of substrates was required, see: (a) A. A. Lamar and K. M. Nicholas, J. Org. Chem., 2010, 75, 7644 CrossRef CAS PubMed; (b) Y. Takeda, J. Hayakawa, K. Yano and S. Minakata, Chem. Lett., 2012, 41, 1672 CrossRef CAS; (c) Y. Amaoka, S. Kamijo, T. Hoshikawa and M. Inoue, J. Org. Chem., 2012, 77, 9959 CrossRef CAS PubMed.
- Hydroxylation followed by Ritter amination of tertiary C–H bonds of alkylamines by methyl(trifluoromethyl)dioxirane in strong acid medium was reported, see: G. Asensio, M. E. González-Núñez, C. B. Bernardini, R. Mello and W. Adam, J. Am. Chem. Soc., 1993, 115, 7250 CrossRef CAS.
- (a) V. R. Koch and L. L. Miller, J. Am. Chem. Soc., 1973, 95, 8631 CrossRef CAS; (b) S. R. Jones and J. M. Mellor, J. Chem. Soc., Perkin Trans. 1, 1976, 2576 RSC; (c) M. Ohsugi, Y. Inamoto, N. Takaishi, Y. Fujikura and K. Aigami, Synthesis, 1977, 632 CrossRef CAS; (d) R. D. Bach, J. W. Holubka, R. C. Badger and S. J. Rajan, J. Am. Chem. Soc., 1979, 101, 4416 CrossRef CAS; (e) J. M. Bakke and C. B. Storm, Acta Chem. Scand., 1989, 43, 399 CrossRef CAS; (f) G. A. Olah and Q. Wang, Synthesis, 1992, 1090 CrossRef CAS; (g) G. A. Olah, P. Ramaiah, C. B. Rao, G. Sandford, R. Golam, N. J. Trivedi and J. A. Olah, J. Am. Chem. Soc., 1993, 115, 7246 CrossRef CAS; (h) C. L. Hill, Synlett, 1995, 127 CrossRef CAS.
- S. Sakaguchi, T. Hirabayashi and Y. Ishii, Chem. Commun., 2002, 516 RSC.
- Q. Michaudel, D. Thevenet and P. S. Baran, J. Am. Chem. Soc., 2012, 134, 2547 CrossRef CAS PubMed.
- (a) J. Buddrus and H. Plettenberg, Angew. Chem., Int. Ed., 1976, 15, 436 CrossRef; (b) J. Gallos and A. Varvoglis, J. Chem. Soc., Perkin Trans. 1, 1983, 1999 RSC; (c) J. Barluenga, F. González-Bobes and J. M. González, Angew. Chem., Int. Ed., 2002, 41, 2556 CrossRef CAS; (d) C. Martínez and K. Muñiz, Angew. Chem., Int. Ed., 2015, 54, 8287 CrossRef PubMed.
- See the ESI† for details.
- For a review, see: (a) A. G. Choghamarani, Synlett, 2006, 2347 CrossRef CAS . For selected examples of reactions using HIO3 and I2O5, see: ; (b) M. J. Cohen and E. McNelis, J. Org. Chem., 1984, 49, 515 CrossRef CAS; (c) K. Yoshida, J. Goto and Y. Ban, Chem. Pharm. Bull., 1987, 35, 4700 CrossRef CAS; (d) M. M. Lakouraj, M. Tajbakhsh, F. Shirini and M. V. Asady Tamami, Synth. Commun., 2005, 35, 775 CrossRef CAS; (e) P. D. Salgaonkar, V. G. Shukla and K. G. Akamanchi, Synth. Commun., 2005, 35, 2805 CrossRef CAS; (f) K. C. Nicolaou, T. Montagnon and P. S. Baran, Angew. Chem., Int. Ed., 2002, 41, 1386 CrossRef CAS; (g) S. Chandrasekhar and K. Gopalaiah, Tetrahedron Lett., 2002, 43, 4023 CrossRef CAS; (h) L. Chai, Y. Zhao, Q. Sheng and Z.-Q. Liu, Tetrahedron Lett., 2006, 47, 9283 CrossRef CAS; (i) Z.-Q. Liu, Y. Zhao, H. Luo, L. Chai and Q. Sheng, Tetrahedron Lett., 2007, 48, 3017 CrossRef CAS; (j) J. Wu, G. Wu and L. Wu, Synth. Commun., 2008, 38, 2367 CrossRef CAS; (k) Z. Hang, Z. Li and Z.-Q. Liu, Org. Lett., 2014, 16, 3648 CrossRef CAS PubMed; (l) L. Zhang, Z. Li and Z.-Q. Liu, Org. Lett., 2014, 16, 3688 CrossRef CAS PubMed; (m) Z. Li, K. Wang and Z.-Q. Liu, Synlett, 2014, 2508 CrossRef CAS.
- Small amount of the corresponding tertiary alcohol, 3-hydroxy-3-methylbutyl benzoate, was generated as a by-product. No amide product 2a was obtained when the alcohol was exposed to HIO3 in MeCN, indicating that the alcohol was not an intermediate of the amination.
- M. S. Chen and M. C. White, Science, 2007, 318, 783 CrossRef CAS PubMed.
- (a) Y. Ishii, T. Iwahama, S. Sakaguchi, K. Nakayama and Y. Nishiyama, J. Org. Chem., 1996, 61, 4520 CrossRef CAS PubMed; (b) N. Koshino, Y. Cai and J. H. Espenson, J. Phys. Chem. A, 2003, 107, 4262 CrossRef CAS; (c) R. Amorati, M. Lucarini, V. Mugnaini, G. F. Pedulli, F. Minisci, F. Recupero, F. Fontana, P. Astolfi and L. Greci, J. Org. Chem., 2003, 68, 1747 CrossRef CAS PubMed; (d) N. Koshino, B. Saha and J. H. Espenson, J. Org. Chem., 2003, 68, 9364 CrossRef CAS PubMed; (e) C. Annunziatini, M. F. Gerini, O. Lanzalunga and M. Lucarini, J. Org. Chem., 2004, 69, 3431 CrossRef CAS PubMed.
- The retention of stereochemical information of the recovered starting material was determined by the specific rotation. See the ESI† for details.
- The iodide 6 was hydrolyzed in situ, and benzoic acid was produced. No product resulting from the amination at the tertiary carbon center was observed.
- V. G. Tsypin, M. S. Pevzner and E. L. Golod, Russ. J. Org. Chem., 2001, 37, 1762 CrossRef CAS.
- The substitution by a nitrile likely proceeds via a cationic intermediate. However, the detail of the mechanism as well as the stereochemical course of the step is not clear at this stage.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data, and spectra. See DOI: 10.1039/c6cc07164c |
| This journal is © The Royal Society of Chemistry 2016 |