Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Water-based synthesis and characterisation of a new Zr-MOF with a unique inorganic building unit†
S.
Waitschat
,
H.
Reinsch
and
N.
Stock
 *
*
Institut für Anorganische Chemie, Christian-Albrechts-Universität, Max-Eyth-Straße 2, D 24118 Kiel, Germany. E-mail: stock@ac.uni-kiel.de
First published on 1st September 2016
Abstract
A new, microporous Zr-MOF was obtained using 2,5-pyrazinedicarboxylic acid (H2PzDC). The linker leads to the formation of a new 1D inorganic building unit composed of μ-OH bridged {Zr6O4(OH4)} clusters which are arranged in a hexagonal array and connected by the PzDC2− ions. The structure was determined from powder X-ray diffraction data.
Metal organic frameworks (MOFs) have been intensively investigated for various applications like gas storage,1 gas separation,2 catalysis,3 heat transformation4 or drug delivery5 due to their high specific surface areas and the diversity of their structures.6
In particular Zr-MOFs are studied for possible applications, due to their thermal and chemical stability.7,8 An outstanding structural feature in carboxylate-based Zr-MOFs is the presence of hexanuclear clusters of composition {Zr6O4(OH4)} which are also well known from molecular Zr(IV) complexes (Fig. 1, bottom, left).9 The clusters are readily formed under various reactions conditions and dominate the structural chemistry of Zr-MOFs. The first example that contains this inorganic building unit (IBU) is UiO-66 ([Zr6O4(OH)4(BDC)6], BDC2− = benzenedicarboxylate, UiO = University of Oslo), which was reported in 2008. In the ideal structure the IBUs are twelvefold connected and form a fcu network topology.10 Isoreticular structures have been reported as well with linkers of different sizes, e.g., DUT-52 (DUT = Dresden University of Technology)11 or UiO-67,10 Zr-Fum,12 ZrSQU13 or for example with functionalized terephthalic acids.14,15 Other topologies have been observed as well, when the hexanuclear IBU is ten (DUT-6916), eight (DUT-67,16 DUT-51,17 MOF-841,18 MOF-54519 or PCN-521, PCN = porous coordination network20) or six connected (MOF-80818). Exceptions where other IBUs are observed are found in the structures of MIL-140 (MIL = Material Institute Lavoisier)21 and PCN-22122 where {Zr(μ3-O)3O4} chains (Fig. 1, top, right) and {Zr8O6} clusters (Fig. 1, middle, left) are incorporated. The {Zr8O6} cluster has been reported once and the structure refinement lead only to rather high R1/wR2 values (0.1924/0.4359 for Zr-PCN-221(Cu)). In the phosphonate- and phenolate-based MOFs UPG-123 (UPG = University of Perugia) and MIL-163,24 ZrO6-polyhedra (Fig. 1, top, left) and edge-sharing ZrO8-polyhedra IBUs (Fig. 1, middle, right) have been observed, respectively (Table S1.3, ESI†).
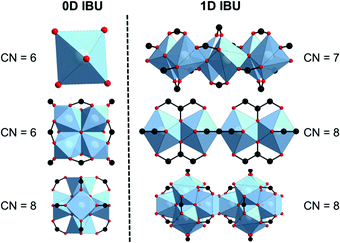 | ||
| Fig. 1 Inorganic building units as observed in various Zr-MOFs.10,21–24 | ||
Zr-MOFs have been mainly synthesized under autogenous pressure using DMF as the solvent,8,10,16,18 however, recently green synthesis routes and reaction upscaling have been reported.25–30 For green syntheses the choice of the metal source and the solvent employed are crucial, and reactions in water, ethanol, acetic or formic acids are usually preferred.
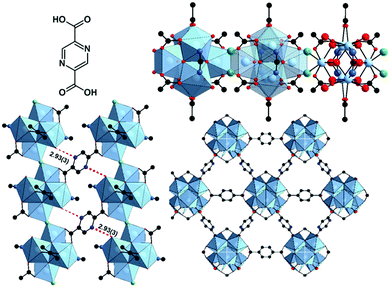
Herein we report the water-based synthesis and detailed characterization of a new Zr-MOF, denoted as CAU-22 (CAU = Christian-Albrechts-Universität) which contains 2,5-pyrazine-dicarboxylate ions (PzDC2−) and a unique 1D IBU of edge-sharing hexanuclear {Zr6O4(OH4)} clusters.
The linker H2PzDC was synthesized as previously reported31 (see S2, ESI†) and to the best of our knowledge it has only been successfully used in the synthesis of a series of lanthanide-MOFs.32,33
The discovery and synthesis optimization of the title compound was carried out in 2 ml Teflon reactors using high-throughput methods as established in our group,34 and setting the reaction time and temperature to 24 h and 120 °C. Initially the influence of the Zr salt on the product formation was evaluated. Using Zr(SO4)2·4H2O and ZrO(NO3)2·xH2O in a solvent mixture of water and formic acid yielded exclusively X-ray amorphous products, while the use of ZrCl4 and ZrOCl2·8H2O resulted in crystalline products. Systematic optimization of the solvent composition (formic acid to H2O ratio) led to a highly crystalline product (Fig. 2). Synthesis details and PXRD patterns are given in the supporting information (Table S3.1 and Fig. S3.1–3.3, ESI†).
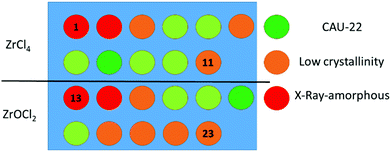 | ||
| Fig. 2 Results of the synthesis optimization by systematically varying the ratio formic acid to H2O from 0% (1, 13) to 100% (11, 23) formic acid. | ||
For both metal sources, ZrCl4 and ZrOCl2·8H2O, an equimolar metal to linker ratio and similar formic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios of 70
H2O ratios of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 for and 50
30 for and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, respectively, results in the formation of the most crystalline reaction product. For full characterization of CAU-22, the synthesis was scaled up in a 30 ml Teflon reactor keeping the optimized reaction conditions constant and using ZrOCl2·8H2O as the metal source, which is much cheaper compared to ZrCl4.
50, respectively, results in the formation of the most crystalline reaction product. For full characterization of CAU-22, the synthesis was scaled up in a 30 ml Teflon reactor keeping the optimized reaction conditions constant and using ZrOCl2·8H2O as the metal source, which is much cheaper compared to ZrCl4.
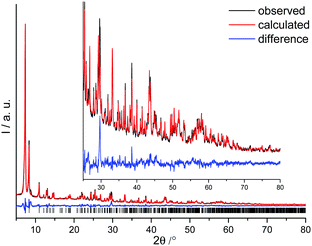
CAU-22 was only obtained as a microcrystalline product and hence the structure had to be determined from PXRD data. Indexing was carried out with Topas35 and the structure was solved by direct methods using the program suit Expo.36 The initial structure model was completed using Material Studio37 and could be successfully refined by Rietveld methods (Fig. 3, see S4, ESI† for details).
CAU-22 (Fig. 4) crystallizes in the monoclinic space group C2/m with the unit cell parameters a = 21.260(2), b = 14.516(2), c = 8.105(2) Å and β = 84.25(2)°. The IBU is based on the very well-known hexanuclear cluster {Zr6O4(OH4)}.10 In this cluster a square antiprismatic coordination of each Zr4+ ion by eight oxygen atoms from O2−, OH− and –CO2− groups is observed. In contrast to all other known Zr-MOFs, in CAU-22 these clusters are condensed into {[Zr6O4(OH)4(μ-OH)2]} chains by edge-bridging OH− ions (Fig. 4, see also ESI†). Each resulting 1D IBUs is connected to six others by PzDC2− linker molecules (Fig. 4) and 1D triangular pores with a diameter of ca. 3 Å are formed, taking the van der Waals radii of the atoms lining the pores into account. These pores are occupied by water molecules. Every hexanuclear subunit is coordinated by six PzDC2− linker molecules and furthermore two formate as well as H2O/OH− molecules capping residual coordination sites (Fig. S4.1–S4.10, ESI†).
The latter has been proposed for many other Zr-MOFs where a connectivity <12 of the clusters is observed.7 Although H2O, OH− and O2− groups cannot be distinguished from the PXRD data, based on the available data and the structure of the hexanuclear clusters, we propose the following sum formula [Zr6(μ3-O)4(μ3-OH)4(μ-OH)2(OH)2(H2O)2(HCO2)2(PzDC)3] for CAU-22, which is also corroborated by additional characterization data.
In addition to the crystal structure analysis, CAU-22 was thoroughly characterised by various methods to confirm the composition and to evaluate the thermal and chemical stabilities as well as the porosity. Temperature dependent powder X-ray diffraction shows a thermal stability for CAU-22 up to approximately 270 °C (Fig. S5.1, ESI†). The thermogravimetric and the elemental analysis (Fig. S5.3 and Table S5.2, ESI† respectively) are in good agreement with the postulated sum formula of the framework [Zr6(μ3-O)4(μ3-OH)4(μ2-OH)2(OH)2(H2O)2(HCO2)2(PzDC)3], but the determined amount of adsorbed water molecules differs due to the hydrophilic properties of CAU-22. The presence of water molecules in the pores of CAU-22 is confirmed by IR-spectroscopy where a strong band centred at 3267 cm−1 is observed (Fig. S6.1, ESI†). The 1H NMR spectrum of the dissolved MOF verifies the molar ratio PzDC2− to formate ions of three to two (Fig. S7.1, ESI†). SEM micrographs of CAU-22 show that the particles consist of small platelets of approximately 0.25 μm in diameter, which are assembled to larger aggregates of octahedral morphology (Fig. S8.1, ESI†).
To determine the porosity of CAU-22 a sample was activated at 120 °C for 16 h under reduced pressure. The nitrogen sorption measurement was carried out at −196 °C. The isotherm shows a Type I behaviour, typical for microporous compounds (Fig. 5), with a small hysteresis (H4-type). The latter is typical for microporous aggregated samples (Fig. S3.6, ESI†).38 The specific surface area is 276 m2 g−1 and the micropore volume is 0.12 cm3 g−1 (determined at p/p0 = 0.5). It is slightly smaller than the theoretical micropore volume of 0.17 cm3 g−1 calculated with the program Platon.39 CAU-22 is highly hydrophilic, as demonstrated by water vapour sorption measurements carried out at 25 °C (Fig. 5).
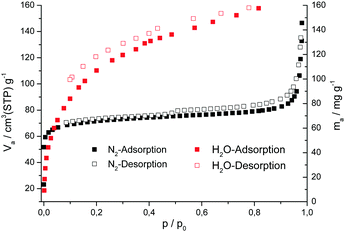 | ||
| Fig. 5 Nitrogen (black) and water (red) sorption isotherms of CAU-22 measured at −196 °C and 25 °C respectively. | ||
The water isotherm shows a steep increase at low relative humidity values and an absolute uptake of about 0.16 g g−1 CAU-22, which is in good agreement with the anticipated pore volume. Compared to UiO-66 (0.30 g g−1) the absolute water uptake is decreased but the steep rise in the isotherm starts at much lower p/p0 values, which can be associated with the more hydrophilic properties of the linker compared to terephthalate ions in UiO-66. After the sorption measurements, the high crystallinity of the samples was demonstrated by powder X-ray diffraction (Fig. S9.2, ESI†).
In conclusion, we synthesised a new Zr-MOF denoted CAU-22 by a water-based synthesis route employing a solvent mixture of water and formic acid. The structure of CAU-22 contains a unique 1D IBU which is formed by edge-sharing of the well-known hexanuclear {[Zr6(μ3-O)4(μ3-OH)4]} clusters. It is permanently porous towards nitrogen and water and In contrast to UiO-66 CAU-22 is very hydrophilic which is due to the incorporation of pyrazinedicarboxylate ions as the linker. We anticipate that other linker molecules with similar functionalities will lead to the same IBU.
We thank Daria Smazna from the technical faculty of the CAU Kiel for taking the SEM micrographs.
Notes and references
- J. A. Mason, M. Veenstra and J. R. Long, Chem. Sci., 2014, 5, 32–51 RSC.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- P. Valvekens, F. Vermoortele and D. De Vos, Catal. Sci. Technol., 2013, 3, 1435–1445 CAS.
- F. Jeremias, D. Frohlich, C. Janiak and S. K. Henninger, New J. Chem., 2014, 38, 1846–1852 RSC.
- F. Ke, Y.-P. Yuan, L.-G. Qiu, Y.-H. Shen, A.-J. Xie, J.-F. Zhu, X.-Y. Tian and L.-D. Zhang, J. Mater. Chem., 2011, 21, 3843–3848 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974–986 CrossRef CAS PubMed.
- Y. Bai, Y. Dou, L.-H. Xie, W. Rutledge, J.-R. Li and H.-C. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC.
- T. Devic and C. Serre, Chem. Soc. Rev., 2014, 43, 6097–6115 RSC.
- M. Puchberger, F. R. Kogler, M. Jupa, S. Gross, H. Fric, G. Kickelbick and U. Schubert, Eur. J. Inorg. Chem., 2006, 3283–3293 CrossRef CAS.
- J. H. Cavka, S. R. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- V. Bon, I. Senkovska, M. S. Weiss and S. Kaskel, CrystEngComm, 2013, 15, 9572–9577 RSC.
- G. Wißmann, A. Schaate, S. Lilienthal, I. Bremer, A. M. Schneider and P. Behrens, Microporous Mesoporous Mater., 2012, 152, 64–70 CrossRef.
- B. Bueken, H. Reinsch, N. Reimer, I. Stassen, F. Vermoortele, R. Ameloot, N. Stock, C. E. A. Kirschhock and D. De Vos, Chem. Commun., 2014, 50, 10055–10058 RSC.
- S. Biswas, J. Zhang, Z. Li, Y.-Y. Liu, M. Grzywa, L. Sun, D. Volkmer and P. Van Der Voort, Dalton Trans., 2013, 42, 4730–4737 RSC.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- V. Bon, I. Senkovska, I. A. Baburin and S. Kaskel, Cryst. Growth Des., 2013, 13, 1231–1237 CAS.
- V. Bon, V. Senkovskyy, I. Senkovska and S. Kaskel, Chem. Commun., 2012, 48, 8407–8409 RSC.
- H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369–4381 CrossRef CAS PubMed.
- W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart and O. M. Yaghi, Inorg. Chem., 2012, 51, 6443–6445 CrossRef CAS PubMed.
- M. Zhang, Y.-P. Chen, M. Bosch, T. Gentle, K. Wang, D. Feng, Z. U. Wang and H.-C. Zhou, Angew. Chem., Int. Ed., 2014, 53, 815–818 CrossRef CAS PubMed.
- V. Guillerm, F. Ragon, M. Dan-Hardi, T. Devic, M. Vishnuvarthan, B. Campo, A. Vimont, G. Clet, Q. Yang, G. Maurin, G. Férey, A. Vittadini, S. Gross and C. Serre, Angew. Chem., Int. Ed., 2012, 51, 9267–9271 CrossRef CAS PubMed.
- D. Feng, H.-L. Jiang, Y.-P. Chen, Z.-Y. Gu, Z. Wei and H.-C. Zhou, Inorg. Chem., 2013, 52, 12661–12667 CrossRef CAS PubMed.
- M. Taddei, F. Costantino, F. Marmottini, A. Comotti, P. Sozzani and R. Vivani, Chem. Commun., 2014, 50, 14831–14834 RSC.
- G. Mouchaham, L. Cooper, N. Guillou, C. Martineau, E. Elkaïm, S. Bourrelly, P. L. Llewellyn, C. Allain, G. Clavier, C. Serre and T. Devic, Angew. Chem., Int. Ed., 2015, 54, 13297–13301 CrossRef CAS PubMed.
- Z. Hu, Y. Peng, Z. Kang, Y. Qian and D. Zhao, Inorg. Chem., 2015, 54, 4862–4868 CrossRef CAS PubMed.
- H. Reinsch, S. Waitschat, S. M. Chavan, K. P. Lillerud and N. Stock, Eur. J. Inorg. Chem., 2016 DOI:10.1002/ejic.201600295.
- F. Ragon, B. Campo, Q. Yang, C. Martineau, A. D. Wiersum, A. Lago, V. Guillerm, C. Hemsley, J. F. Eubank, M. Vishnuvarthan, F. Taulelle, P. Horcajada, A. Vimont, P. L. Llewellyn, M. Daturi, S. Devautour-Vinot, G. Maurin, C. Serre, T. Devic and G. Clet, J. Mater. Chem. A, 2015, 3, 3294–3309 CAS.
- Q. Yang, S. Vaesen, F. Ragon, A. D. Wiersum, D. Wu, A. Lago, T. Devic, C. Martineau, F. Taulelle, P. L. Llewellyn, H. Jobic, C. Zhong, C. Serre, G. De Weireld and G. Maurin, Angew. Chem., Int. Ed., 2013, 52, 10316–10320 CrossRef CAS PubMed.
- H. Reinsch, B. Bueken, F. Vermoortele, I. Stassen, A. Lieb, K.-P. Lillerud and D. De Vos, CrystEngComm, 2015, 17, 4070–4074 RSC.
- H. Reinsch, Eur. J. Inorg. Chem., 2016 DOI:10.1002/ejic.201600286.
- W. J. Schut, H. I. X. Mager and W. Berends, Recl. Trav. Chim. Pays-Bas, 1961, 80, 391–398 CrossRef CAS.
- R. Plessius, R. Kromhout, A. L. D. Ramos, M. Ferbinteanu, M. C. Mittelmeijer-Hazeleger, R. Krishna, G. Rothenberg and S. Tanase, Chem. – Eur. J., 2014, 20, 7922–7925 CrossRef CAS PubMed.
- B. Cai, P. Yang, J.-W. Dai and J.-Z. Wu, CrystEngComm, 2011, 13, 985–991 RSC.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- A. Topas Academics 4.2, Coelho Software, Brisbane, 2007 Search PubMed.
- A. Altomare, M. Camalli, C. Cuocci, C. Giacovazzo, A. Moliterni and R. Rizzi, J. Appl. Crystallogr., 2009, 42, 1197–1202 CrossRef CAS.
- C. Materials Studio Version 5.0, Accelrys Inc., San Diego, 2009 Search PubMed.
- M. Thommes, K. Kaneko, V. Neimark Alexander, P. Olivier James, F. Rodriguez-Reinoso, J. Rouquerol and S. W. Sing Kenneth, Pure Appl. Chem., 2015, 87, 1051 CrossRef CAS.
- A. Spek, Acta Crystallogr., 2009, 65, 148–155 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of high-throughput-syntheses and structure determination, variable temperature PXRD data, TG analysis, IR- and NMR spectra, SEM micrographs. CCDC 1496183 contains the supplementary crystallographic data for CAU-22. See DOI: 10.1039/c6cc06287c |
| This journal is © The Royal Society of Chemistry 2016 |