Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Manipulating pH using near-infrared light assisted by upconverting nanoparticles†
Zhijun
Chen
a,
Yubing
Xiong
a,
Roberto
Etchenique
b and
Si
Wu
*a
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128, Mainz, Germany. E-mail: wusi@mpip-mainz.mpg.de
bDepartamento de Química Inorgánica, Analítica y Química Física, INQUIMAE, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria Pabellón 2, AR1428EHA Buenos Aires, Argentina
First published on 10th October 2016
Abstract
Near-infrared light can be used to manipulate the pH of aqueous solutions by using upconverting nanoparticle-assisted photocleavage of a ruthenium complex photobase. Upconverting nanoparticles and the photobase were also introduced into a pH-responsive hydrogel, in which near-infrared irradiation induced swelling of the hydrogel.
pH is an important parameter in many chemical, physical and biological processes.1,2 Photoacids/bases, which can decrease/increase pH upon light irradiation, enable remote control of pH with high spatiotemporal resolution.3 Light-induced pH change can further control deformation of hydrogels,4 conductivity,5 polymerization,6 and host–guest interactions.7 pH manipulation has been proposed as a powerful technique to achieve control over relevant paths related to several diseases such as cancer, cardiovascular disease, Alzheimer's disease, etc.8 However, most photoacids/bases are sensitive to only UV light which can damage biological systems.9–11 Recently, Liao et al. reported visible-light-responsive photoacids.4,8,12,13 Further, one of their photoacids can be used in PBS buffer,8 which is desirable for biomedical applications. Nevertheless, visible light is still not able to deeply penetrate into tissue.14 Compared to UV and visible light, near-infrared (NIR) light is better suited for biomedical applications because NIR light causes less photodamage to biological systems and can penetrate much deeper into tissue.15 Therefore, developing NIR light-induced pH manipulation represents significant progress for the biomedical field.
A promising approach to NIR light-induced pH manipulation is based on photochemistry assisted by lanthanide-doped upconverting nanoparticles (UCNPs). UCNPs can convert NIR light into UV/visible light.16,17 The upconverted UV/visible light can then induce photoreactions of conventional UV-/visible-light-sensitive compounds. This process is called UCNP-assisted photochemistry.18–21 UCNP-assisted photoisomerization,22,23 photocleavage,24–26 photopolymerization,27,28 and photocoupling reaction29 have already been studied in the context of various applications.30–37 Additionally, a new type of UCNP-assisted photochemical process, “UCNP-assisted photoinduced protonation/deprotonation”, was proposed in the outlook of a recent review.33 These previous works inspired us to use NIR light to control pH assisted by UCNPs.
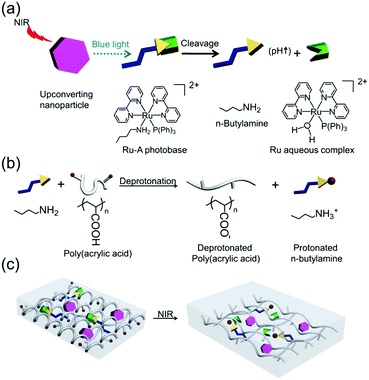
Here, we experimentally demonstrate that the combination of UCNPs and a photobase enables the control of pH by NIR light (Fig. 1a). We refer to this new combination as a photon upconversion pH manipulation. The complex [Ru(bpy)2(PPh3)(BuNH2)]2+ (bpy = 2,2′ bipyridine, PPh3 = triphenylphosphine, BuNH2 = n-butylamine), hereafter Ru-A (Fig. 1a), was used as the photobase because blue light irradiation on Ru-A can cause a pH change within nano to microseconds.38 Moreover, we found that UCNPs can efficiently assist photocleavage of Ru complexes because of spectral overlap of Ru complex absorption39–41 and UCNP emission.19,42 Further, we have recently demonstrated the true sectioning power of the upconversion excitation,17 allowing precise z-axis manipulation of the downstream effects of NIR light irradiation. On these bases, Ru-A was combined with UCNPs, which would convert NIR light into blue light to enable the release of n-butylamine from Ru-A (Fig. 1a). The released butylamine would then increase the pH of an aqueous solution. When NIR light-induced release of n-butylamine occurs in the presence of poly(acrylic acid) (PAA), PAA can become more hydrophilic via deprotonation and thus swell due to electrostatic repulsion (Fig. 1b). Thus, to demonstrate the potential application of NIR light-manipulated pH, we introduced UCNPs and Ru-A into a PAA hydrogel (Fig. 1c).
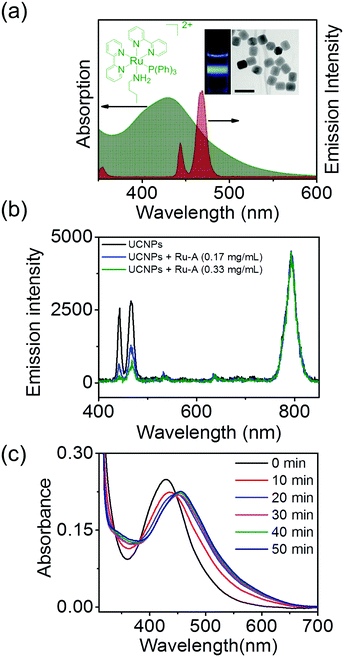
NaYF4:TmYb@NaYF4 UCNPs (core = NaYF4: 0.5 mol% Tm3+: 30 mol% Yb3+; shell = NaYF4) with a diameter of 50 nm were synthesized as the upconverters (Fig. 2a). These UCNPs emitted blue light under 974 nm NIR light excitation. The absorption band of the photobase Ru-A in the blue light region overlapped with the blue emission of the UCNPs (Fig. 2a). To demonstrate absorption of upconverted blue light by Ru-A, we compared the upconversion luminescence spectra of UCNPs in the presence and absence of Ru-A. The intensity of upconversion luminescence of UCNPs at 440 nm and 470 nm decreased significantly in the presence of Ru-A (Fig. 2b). Additionally, Ru-A with higher concentration absorbed more upconverted blue light and resulted in lower emission intensity. In contrast, the emission at 800 nm, a spectral region where Ru-A has no absorption, still remained (Fig. 2b). This result proved efficient absorption of the upconverted blue light by Ru-A. When irradiating a dispersion of UCNPs and Ru-A with NIR light, the absorption band of Ru-A decreased and red shifted (Fig. 2c). This spectral change was identical to that observed for Ru-A which was directly photocleaved using blue light (Fig. S1, ESI†). Thus, Ru-A was photocleaved through NIR light irradiation. The exposure of Ru-A to NIR light in the absence of UCNPs did not change the absorption spectrum (Fig. S2, ESI†), proving that the photocleavage of Ru-A was induced by the photon upconversion process.
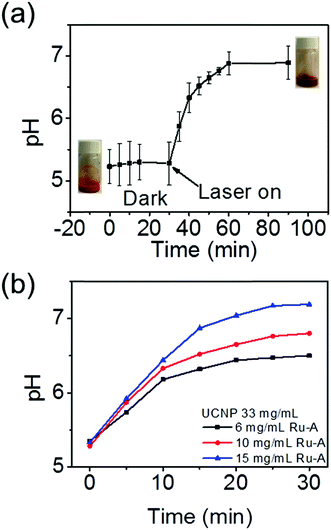
To demonstrate a NIR light-induced pH increase, an aqueous dispersion of UCNPs was prepared by ultrasonic treatment of UCNPs in water (Fig. S3 and Video 1, ESI†). Afterwards, Ru-A was introduced into the UCNP dispersion. The UCNP/Ru-A dispersion was placed into an ice bath and irradiated with NIR light. The NIR light-induced pH change of the UCNP/Ru-A dispersion was measured (Fig. 3a). The initial pH of the UCNP/Ru-A dispersion was 5.2, which did not change in the dark. However, exposure to NIR light (5.5 W cm−2) changed the pH from 5.23 to 6.80 (Fig. 3a). NIR light irradiation can induce the release of n-butylamine from Ru-A (Fig. 2). The coordinated n-butylamine in Ru-A does not act as a base because its electron pair is strongly coordinated to Ru2+. However, the released free n-butylamine is a relatively strong base with pKa 10.77.38 Thus, the NIR light-induced pH change was attributed to the released n-butylamine from Ru-A. As a control experiment, exposure of Ru-A in the absence of UCNPs to NIR light in an ice bath showed no pH change (Fig. S4, ESI†), which further confirmed that the pH change was due to the photon upconversion process. In addition, NIR light irradiation also changed the color of the dispersion because the absorption spectra of Ru-A and the Ru-aqua photoproduct (Ru–H2O, Fig. 1a) are different. Moreover, the ratio of UCNPs to Ru-A was tuned to investigate its influence on the pH change of the solution. The concentration of UCNPs was fixed. As the concentration of Ru-A increased, the pH change was larger (Fig. 3b). The larger pH change was because more n-butylamine could be released from Ru-A with a higher concentration. Thus, the ratio of UCNPs to Ru-A can be used to adjust the range of pH change.
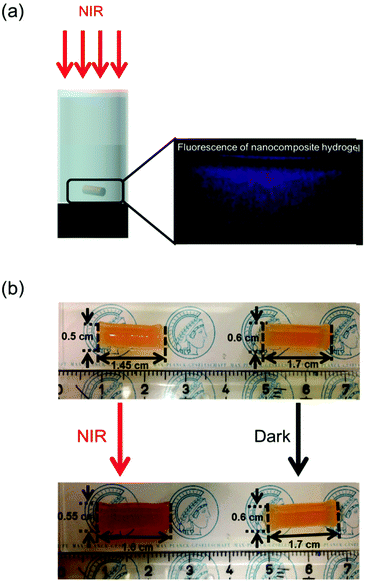
Subsequently, the NIR light-manipulated pH increase was used to control deformation of a pH-sensitive hydrogel. A nanocomposite hydrogel was prepared by cross-linking PAA in the presence of Ru-A and UCNPs. Upconverted luminescence was observed from the nanocomposite hydrogel upon irradiation with NIR light (Fig. 4a), which further confirmed that UCNPs were incorporated inside the hydrogel. A nanocomposite hydrogel with a volume of 0.28 cm3 was immersed in an aqueous solution (pH 3.5) and irradiated with NIR light for 15 min in an ice bath. NIR light irradiation was conducted in an ice bath because an ice bath can prevent overheating problems of NIR light irradiation and heat-induced side effects (Fig. S5 and S6, ESI†).33,43 After irradiation, the volume of the hydrogel increased to 0.37 cm3, which was 32% greater than the hydrogel before irradiation (Fig. 4b, left). In addition, the color of the hydrogel changed from orange to brown upon NIR light irradiation, which is identical to the color change observed in the dispersion of UCNPs and Ru-A upon NIR light irradiation (Fig. 3a). Thus, the swelling was attributed to the deprotonation of PAA by the n-butylamine released from Ru-A. In a control experiment, the hydrogel without irradiation did not change in size or color (Fig. 4b, right). In another two control experiments, hydrogels with only Ru-A or UCNPs were prepared. The hydrogel with Ru-A and without UCNPs did not show any color or volume change upon NIR light irradiation in an ice bath (Fig. S7, ESI†). The hydrogel incorporating only UCNPs was also unresponsive to NIR light irradiation (Fig. S8, ESI†). These results further confirmed that the swelling of the hydrogel was due to the released n-butylamine upon NIR light irradiation.
In conclusion, we demonstrated that NIR light could increase the pH with the assistance of UCNPs and the photobase Ru-A. UCNPs converted NIR light into blue light, which triggered the release of n-butylamine from Ru-A. The released n-butylamine could further deprotonate PAA. The strategy of photon upconversion pH manipulation was further developed to control the swelling of pH-sensitive PAA hydrogels. Not only Ru-A but many other photoacids/photobases can also alter solution pH after light irradiation. Thus, the concept “photon upconversion pH manipulation” reported in this work is a general approach to controlling pH by NIR light. Also, photon upconversion pH manipulation can not only induce swelling of pH-sensitive hydrogels but can also stimulate other pH-responsive materials, such as micelles, capsules, and supramolecules. Thus, photon upconversion pH manipulation is a new and general way to control pH-responsive materials with high spatiotemporal resolution for various applications.
Z. C. and Y. X. were supported by the CSC program. R. E. is a member of CONICET. We thank H. Menges for measuring the upconverting luminescence spectra. This work was partly supported by the Deutsche Forschungsgemeinschaft (DFG, WU 787/2-1).
Notes and references
- H. Sohn, S. Létant, M. J. Sailor and W. C. Trogler, J. Am. Chem. Soc., 2000, 122, 5399 CrossRef CAS.
- Y. Bae, S. Fukushima, A. Harada and K. Kataoka, Angew. Chem., Int. Ed., 2003, 42, 4640 CrossRef CAS PubMed.
- M. Shirai and M. Tsunooka, Prog. Polym. Sci., 1996, 21, 1 CrossRef CAS.
- Z. Shi, P. Peng, D. Strohecker and Y. Liao, J. Am. Chem. Soc., 2011, 133, 14699 CrossRef CAS PubMed.
- S. Haghighat, S. Ostresh and J. M. Dawlaty, J. Phys. Chem. B, 2016, 120, 1002 CrossRef PubMed.
- C. Fu, J. Xu and C. Boyer, Chem. Commun., 2016, 52, 7126 RSC.
- J. Vázquez, M. A. Romero, R. N. Dsouza and U. Pischel, Chem. Commun., 2016, 52, 6245 RSC.
- N. Abeyrathna and Y. Liao, J. Am. Chem. Soc., 2015, 137, 11282 CrossRef CAS PubMed.
- M. Shirai and H. Okamura, Prog. Org. Coat., 2009, 64, 175 CrossRef CAS.
- W. Feng, W. Zhou, S. Zhang, Y. Fan, A. Yasin and H. Yang, RSC Adv., 2015, 5, 81784 RSC.
- A. Chemtob, F. Courtecuisse, C. Croutxé-Barghorn and S. Rigolet, New J. Chem., 2011, 35, 1803 RSC.
- V. K. Johns, P. Peng, J. DeJesus, Z. Wang and Y. Liao, Chem. – Eur. J., 2014, 20, 689 CrossRef CAS PubMed.
- Z. Z. Wang, V. K. Johns and Y. Liao, Chem. – Eur. J., 2014, 20, 14637 CrossRef CAS PubMed.
- P. Juzenas, A. Juzeniene, O. Kaalhus, V. Iani and J. Moan, Photochem. Photobiol. Sci., 2002, 1, 745 CAS.
- R. Wang and F. Zhang, J. Mater. Chem. B, 2014, 2, 2422 RSC.
- C. Li, D. Yang, P. Ma, Y. Chen, Y. Wu, Z. Hou, Y. Dai, J. Zhao, C. Sui and J. Lin, Small, 2013, 9, 4150 CrossRef CAS PubMed.
- J. Hodak, Z. Chen, S. Wu and R. Etchenique, Anal. Chem., 2016, 88, 1468 CrossRef CAS PubMed.
- E. Ruggiero, A. Habtemariam, L. Yate, J. C. Mareque-Rivas and L. Salassa, Chem. Commun., 2014, 50, 1715 RSC.
- Z. Chen, W. Sun, H. J. Butt and S. Wu, Chem. – Eur. J., 2015, 21, 9165 CrossRef CAS PubMed.
- B. Yan, J.-C. Boyer, D. Habault, N. R. Branda and Y. Zhao, J. Am. Chem. Soc., 2012, 134, 16558 CrossRef CAS PubMed.
- B. Yan, J.-C. Boyer, N. R. Branda and Y. Zhao, J. Am. Chem. Soc., 2011, 133, 19714 CrossRef CAS PubMed.
- J. C. Boyer, C. J. Carling, B. D. Gates and N. R. Branda, J. Am. Chem. Soc., 2010, 132, 15766 CrossRef CAS PubMed.
- W. Wu, L. M. Yao, T. S. Yang, R. Y. Yin, F. Y. Li and Y. L. Yu, J. Am. Chem. Soc., 2011, 133, 15810 CrossRef CAS PubMed.
- Y. M. Yang, Q. Shao, R. R. Deng, C. Wang, X. Teng, K. Cheng, Z. Cheng, L. Huang, Z. Liu, X. G. Liu and B. G. Xing, Angew. Chem., Int. Ed., 2012, 51, 3125 CrossRef CAS PubMed.
- J. Shen, G. Chen, T. Y. Ohulchanskyy, S. J. Kesseli, S. Buchholz, Z. Li, P. N. Prasad and G. Han, Small, 2013, 9, 3213 CrossRef CAS PubMed.
- M. L. Viger, M. Grossman, N. Fomina and A. Almutairi, Adv. Mater., 2013, 25, 3733 CrossRef CAS PubMed.
- S. Beyazit, S. Ambrosini, N. Marchyk, E. Palo, V. Kale, T. Soukka, B. T. S. Bui and K. Haupt, Angew. Chem., Int. Ed., 2014, 53, 8919 CrossRef CAS PubMed.
- A. Stepuk, D. Mohn, R. N. Grass, M. Zehnder, K. W. Krämer, F. Pellé, A. Ferrier and W. J. Stark, Dent. Mater., 2012, 28, 304 CrossRef CAS PubMed.
- P. Lederhose, Z. J. Chen, R. Müller, J. P. Blinco, S. Wu and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2016, 55, 12195 CrossRef CAS PubMed.
- D. Yang, P. Ma, Z. Hou, Z. Cheng, C. Li and J. Lin, Chem. Soc. Rev., 2015, 44, 1416 RSC.
- X. G. Liu, C. H. Yan and J. A. Capobianco, Chem. Soc. Rev., 2015, 44, 1299 RSC.
- Z. Y. Cheng and J. Lin, Macromol. Rapid Commun., 2015, 36, 790 CrossRef CAS PubMed.
- S. Wu and H. J. Butt, Adv. Mater., 2016, 28, 1208 CrossRef CAS PubMed.
- A. E. Pierri, P.-J. Huang, J. V. Garcia, J. G. Stanfill, M. Chui, G. Wu, N. Zheng and P. C. Ford, Chem. Commun., 2015, 51, 2072 RSC.
- P. T. Burks, J. V. Garcia, R. GonzalezIrias, J. T. Tillman, M. Niu, A. A. Mikhailovsky, J. Zhang, F. Zhang and P. C. Ford, J. Am. Chem. Soc., 2013, 135, 18145 CrossRef CAS PubMed.
- C. Wang, X. Li and F. Zhang, Analyst, 2016, 141, 3601 RSC.
- J. Zhou, Z. Liu and F. Li, Chem. Soc. Rev., 2012, 41, 1323 RSC.
- O. Filevich, G. Carrone, V. A. Pavlovsky and R. Etchenique, Anal. Chem., 2012, 84, 5618 CrossRef CAS PubMed.
- S. Bonnet, B. Limburg, J. D. Meeldijk, R. J. M. K. Gebbink and J. A. Killian, J. Am. Chem. Soc., 2011, 133, 252 CrossRef CAS PubMed.
- A. Bahreman, B. Limburg, M. A. Siegler, R. Koning, A. J. Koster and S. Bonnet, Chem. – Eur. J., 2012, 18, 10271 CrossRef CAS PubMed.
- J. D. Knoll and C. Turro, Coord. Chem. Rev., 2015, 282–283, 110 CrossRef CAS PubMed.
- S. Q. He, K. Krippes, S. Ritz, Z. J. Chen, A. Best, H. J. Butt, V. Mailänder and S. Wu, Chem. Commun., 2015, 51, 431 RSC.
- J. Y. Dong and J. I. Zink, Small, 2015, 11, 4165 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc05287h |
| This journal is © The Royal Society of Chemistry 2016 |