Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct amidation of unprotected amino acids using B(OCH2CF3)3†
Rachel M.
Lanigan‡
a,
Valerija
Karaluka‡
a,
Marco T.
Sabatini‡
a,
Pavel
Starkov§
 a,
Matthew
Badland
b,
Lee
Boulton
c and
Tom D.
Sheppard
*a
a,
Matthew
Badland
b,
Lee
Boulton
c and
Tom D.
Sheppard
*a
aDepartment of Chemistry, University College London, Christopher Ingold Laboratories, 20 Gordon St, London, WC1H 0AJ, UK. E-mail: tom.sheppard@ucl.ac.uk
bPfizer Global Pharmaceutical Sciences, Discovery Park, Ramsgate Road, Sandwich, Kent CT13 9NJ, UK
cGlaxoSmithKline, Medicines Research Centre, Gunnels Wood Road, Stevenage, Herts SG1 2NY, UK
First published on 22nd June 2016
Abstract
A commercially available borate ester, B(OCH2CF3)3, can be used to achieve protecting-group free direct amidation of α-amino acids with a range of amines in cyclopentyl methyl ether. The method can be applied to the synthesis of medicinally relevant compounds, and can be scaled up to obtain gram quantities of products.
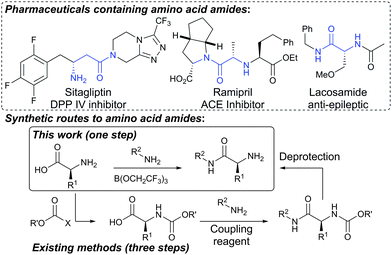
Amino acids are valuable renewable chemical building blocks for the synthesis of a wide range of natural products, pharmaceuticals and agrochemicals, and can serve as a source of chirality for a range of ligands and catalysts. However, their use in synthesis relies heavily on protecting group chemistry,1 with either the amine or the carboxylic acid requiring protection before key bond-forming processes are carried out. Amidation is one of the most common transformations used in the synthesis of many industrially important molecules,2 and amino acid amides form part of the core of numerous globally important pharmaceuticals including sitagliptin, ramipril and lacosamide (Scheme 1).3 The synthesis of amino acid amides typically proceeds via a three step sequence involving protection of the nitrogen atom with a carbamate (Boc, Fmoc, Cbz) before amidation of the carboxylic acid and subsequent deprotection. Nitrogen protection is usually essential as self-reaction of the free amino acid can lead to the formation of diketopiperazines, oligomers or polymers. The use of carbamate protecting groups is highly inefficient as they have a relatively high molecular weight in comparison to the amino acid itself (Boc 101; Fmoc 223; Cbz 135), and their introduction and removal requires additional reagents, solvents and purification materials. A more straightforward approach would be the direct amidation of the free amino acid to give the amino amide in a single step. This has rarely been achieved, however, with only scattered reports in the literature that have not been widely applied.4 The only reasonably effective method involves reaction of the amino acid with the highly toxic gas hexafluoroacetone, followed by subsequent reaction with an amine.5 Although great progress has been made in recent years on the development of new amidation methods6 which make use of low-cost reagents7,8 or catalysts9 that are easy to separate from the amide products, few of these methods are applicable to highly functionalized compounds and none have been applied to the direct amidation of free amino acids.10
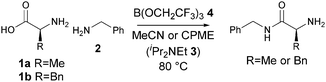
We have recently reported the use of borate esters, and B(OCH2CF3)311 in particular, for the direct amidation of a wide range of acids and amines, including functionalized examples.8 An important advantage of this method is the ease with which the amide products can be purified using a simple filtration work-up involving commercially available resins, which scavenge unreacted acid and amine as well as any boron-containing byproducts. In this paper, we demonstrate that B(OCH2CF3)3 is a highly effective reagent for the direct amidation of unprotected amino acids to give useful α-amino amide products in a single step. This has the potential to significantly shorten the synthesis of many pharmaceutically relevant compounds.
We began our investigation (Scheme 2 and Table 1) by exploring the direct amidation of alanine 1a with benzylamine 2 (entry 1). Under our previous amidation conditions (MeCN, reflux),8 addition of a base (diisopropylethylamine 3) was necessary to solubilise alanine in the reaction. We observed a promising 35% conversion to the desired amide using one equivalent of B(OCH2CF3)34, with little evidence for significant self-reaction of the amino acid. Addition of excess borate 4 increased the conversion to 71% (entry 2). Crucially, in the absence of the borate reagent no conversion to the amino amide was observed (entry 3). As the amidation product derived from alanine was extremely polar and time-consuming to isolate, we switched our attention to the direct amidation of phenylalanine 1b with benzylamine 2. Under similar conditions, this amide was obtained in 61% yield (entry 4). A screen of different solvents,12 led us to identify cyclopentyl methyl ether (CPME)13 as a promising solvent for the amidation reaction (entry 5). A significant improvement in the yield was seen by increasing the number of equivalents of benzylamine 2 used (entry 6), and with excess benzylamine an additional base was no longer required. The importance of using B(OCH2CF3)3 as the coupling reagent was underlined by the fact that B(OMe)38a led to only 9% conversion under otherwise identical reaction conditions (entry 7). As observed previously with alanine, no product was formed in the absence of the borate reagent 4 (entry 8).
| Entry | R | Solvent | 2 (eq.) | 3 (eq.) | 4 (eq.) | Yielda |
|---|---|---|---|---|---|---|
| a Yields measured by 1H NMR using naphthalene as an internal standard; conversions are shown in parentheses. b B(OMe)3 was used instead of 4. | ||||||
| 1 | Me | MeCN | 1 | 1 | 1 | (35) |
| 2 | Me | MeCN | 1 | 1 | 3 | (71) |
| 3 | Me | MeCN | 1 | 1 | 0 | (0) |
| 4 | Bn | MeCN | 1 | 1 | 3 | 61 |
| 5 | Bn | CPME | 1 | 1 | 3 | 65 |
| 6 | Bn | CPME | 3 | 0 | 3 | 95 |
| 7 | Bn | CPME | 3 | 0 | 3b | (9) |
| 8 | Bn | CPME | 3 | 0 | 0 | (0) |
With an effective procedure in hand, we then went on to examine the scope with regard to the amino acid component (Scheme 3). In order to facilitate purification of the products we used n-propylamine as the amine so the products could be purified via a simple solid-phase work-up and subsequent evaporation of the volatile materials. The direct amidation products 5a–5b derived from alanine and phenylalanine were obtained in 71% and 90% isolated yield respectively. Other amino acids containing alkyl/aryl side chains including glycine, valine, leucine, proline and isoleucine also underwent direct amidation in high yield (5c–5g), although in the case of isoleucine a significant amount of epimerization was observed, which could be reduced by reduction of the reaction time and by adding the borate reagent dropwise.
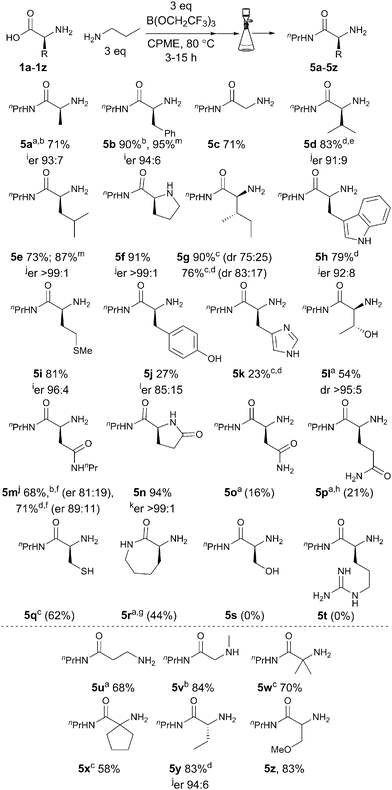 | ||
Scheme 3 Direct amidation of unprotected amino acids with propylamine; isolated yields (NMR yields shown in parentheses);14a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) reaction carried out in MeCN; b reaction carried out in MeCN; b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isolated as HCl salt; c isolated as HCl salt; c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 °C; d 125 °C; d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) borate 4 was added dropwise over 1 h; e borate 4 was added dropwise over 1 h; e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 °C; f 110 °C; f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 eq. of 4 and 6 eq. propylamine; g 4 eq. of 4 and 6 eq. propylamine; g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) no propylamine; h no propylamine; h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15% yield of 5n also observed; i 15% yield of 5n also observed; i![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) determined using imine formation with a chiral aldehyde;15j determined using imine formation with a chiral aldehyde;15j![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) determined using Marfey's reagent;16k determined using Marfey's reagent;16k![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) determined using a chiral shift reagent;17l determined using a chiral shift reagent;17l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) determined by HPLC of a derivative using a chiral stationary phase; m determined by HPLC of a derivative using a chiral stationary phase; m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 g scale. 1 g scale. | ||
Pleasingly, tryptophan (5h) and methionine (5i) were also good substrates for the direct amidation reaction, giving high yields of the corresponding amides. Even amino acids with functionalized side chains were compatible with the reaction conditions, with the amides derived from tyrosine (5j) and histidine (5k) being obtained in moderate yield, and threonine undergoing direct amidation in an impressive yield of 54%, despite the presence of the free hydroxyl group (5l). Aspartic acid underwent double amidation in the presence of excess amine/borate to give the diamide 5m,18 whereas in the case of glutamic acid, lactam formation took place alongside amidation to give 5n. A small improvement in both the yield and enantiopurity was seen by adding the borate dropwise to the reaction mixture. Asparagine and glutamine were poor substrates for the reaction giving only low conversions to the desired amides 5o–5p. Whilst reaction of cysteine led to a good conversion to the desired amide 5q, we were unable to purify this amide despite numerous attempts. Lactamisation of lysine outcompeted the desired amidation reaction with 5r being the only product observed either in the presence or absence of propylamine. Direct amidation of serine and arginine did not give any of the expected amino amide. The reaction of serine led to a complex mixture of products with no observable amide 5s, a surprising result given the successful amidation of threonine. Arginine was insoluble in the reaction mixture and no 5t was observed. Non-proteinogenic amino acids also underwent direct amidation under these conditions (5u–5z) with β-alanine, sarcosine and α-aminoisobutyric acid giving the corresponding amides 5u–5w in good yield. Relatively hindered cyclopentyl amino acid amide 5x was obtained in moderate yield, whereas 2-aminobutyric acid and O-methylserine gave the corresponding amides 5y–5z in excellent yield. Pleasingly, the vast majority of the amides 5a–5z were obtained in high purity from a simple filtration without the need for chromatography or an aqueous work-up. Furthermore, scale-up of two amidation reactions (5b, 5e) to 1 g scale resulted in improved yield of products whilst maintaining the same level of enantiopurity, demonstrating the synthetic utility of this procedure. Whilst a small degree of racemisation was observed with some amino acids, it should be noted that the amino amides (or simple derivatives such as their HCl salts) are usually crystalline so recrystallization could potentially be used to obtain enantiopure material if needed.
The scope of the reaction with regard to the amine component was investigated next (Scheme 4), through the preparation of amides derived from a selection of different amines. Simple aliphatic amines (6a–6f) including an unsaturated example (6c) worked well in the reaction. Amides could also be prepared from cyclic secondary amines in good yield (6g–6j). Importantly, it was also possible to prepare two dipeptide derivatives using amino acid tert-butyl esters as the nucleophile (6k–6l). The methodology was then applied to the synthesis of the previously reported anti-convulsant amino amides 6m–6n19 to demonstrate the application of the methodology. Finally, a synthesis of the anti-epileptic lacosamide 7 was carried out by direct amidation of (R)-1z with benzylamine, followed by acylation with acetic anhydride.20
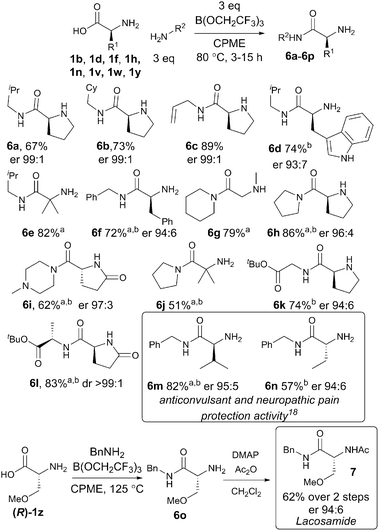 | ||
Scheme 4 Direct amidation reactions of amino acids with various amines; a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 °C; b 125 °C; b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) borate 4 was added dropwise over 1 h. borate 4 was added dropwise over 1 h. | ||
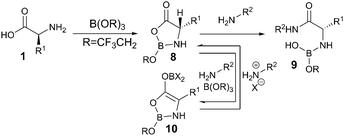
A plausible mechanism for the amidation reactions is shown in Scheme 5. Coordination of both the amine and the carboxylic acid to the borate reagent should lead to cyclic intermediate 8.4a,b This can then undergo reaction with the amine partner to form the boron-bound amino amide 9. In cases where the amino acid and/or the amine are less reactive, racemisation could occur via borate-assisted deprotonation of intermediate 8 by the amine, leading to the formation of the formally aromatic heterocyclic enolate 10.
In summary, we have developed a practical and general method for the protecting-group free direct amidation of free amino acids. The amidation reaction is successful with 14 of the 20 common proteinogenic amino acids as well as six unnatural amino acids, and a variety of amines can be employed. In the majority of cases, the amino acid amide products can be isolated using a simple filtration work-up without the need for chromatographic purification. The utility of the method has been demonstrated by the synthesis of some medicinally relevant compounds.
We would like to thank the Engineering and Physical Sciences Research Council (EPSRC Advanced Research Fellowship to TDS; studentship to PS; Grant reference EP/E052789/1), the Department of Chemistry, University College London (studentship funding to RML and MS), Pfizer (EPSRC CASE award to VK) and GlaxoSmithKline (studentship support to MS). We would also like to acknowledge the EPSRC National Mass Spectrometry Facility at Swansea University for providing analytical services.
Notes and references
-
(a) I. S. Young and P. S. Baran, Nat. Chem., 2009, 1, 193–205 CrossRef CAS PubMed
; (b) T. Newhouse, P. S. Baran and R. W. Hoffmann, Chem. Soc. Rev., 2009, 39, 3010–3021 RSC
.
-
(a) J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC
; (b) L. Amarnath, I. Andrews, R. Bandichhor, A. Bhattacharya, P. J. Dunn, J. Hayler, W. Hinkley, N. Holub, D. Hughes, L. Humphreys, B. Kaptein, H. Krishnen, K. Lorenz, S. Mathew, G. Nagaraju, T. Rammeloo, P. Richardson, L. Wang, A. Wells and T. White, Org. Process Res. Dev., 2012, 16, 535–544 CrossRef
; (c) D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer Jr., R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC
.
-
(a) G. A. Herman, C. Stevens, K. Van Dyck, A. Bergman, B. Yi, M. De Smet, K. Snyder, D. Hilliard, M. Tanen, W. Tanaka, A. Q. Wang, W. Zeng, D. Musson, G. Winchell, M. J. Davies, S. Ramael, K. M. Gottesdiener and J. A. Wagner, Clin. Pharmacol. Ther., 2005, 78, 675–688 CrossRef CAS PubMed
; (b) L. Pilote, M. Abrahamowicz, M. Eisenberg, K. Humphries, H. Behlouli and J. V. Tu, Can. Med. Assoc. J., 2008, 178, 1303–1311 CrossRef PubMed
; (c) A. C. Errington, T. Stöhr, C. Heers and G. Lees, Mol. Pharmacol., 2008, 73, 157–169 CrossRef CAS PubMed
.
-
(a) S. H. van Leeuwen, P. J. L. M. Quaedflieg, Q. B. Broxterman, Y. Milhajlovica and R. M. J. Liskamp, Tetrahedron Lett., 2005, 46, 653–656 CrossRef CAS
; (b) S. H. van Leeuwen, P. J. L. M. Quaedflieg, Q. B. Broxterman and R. M. J. Liskamp, Tetrahedron Lett., 2002, 43, 9203–9207 CrossRef CAS
; (c) J. Li, K. Subramaniam, D. Smith, J. X. Qiao, J. J. Li, J. Qian-Cutrone, J. F. Kadow, G. D. Vite and B.-C. Chen, Org. Lett., 2012, 14, 214–217 CrossRef PubMed
; (d) R. Sharma and R. Jain, Synlett, 2007, 603–606 CAS
; (e) M. A. Schmidt, E. A. Reiff, X. Qian, C. Hang, V. C. Truc, K. J. Natalie, C. Wang, J. Albrecht, A. G. Lee, E. T. Lo, Z. Guo, A. Goswami, S. Goldberg, J. Pesti and L. T. Rossano, Org. Process Res. Dev., 2015, 19, 1317–1322 CrossRef CAS
.
- J. Spengler, C. Böttcher, F. Albericio and K. Burger, Chem. Rev., 2006, 106, 4728–4746 CrossRef PubMed
.
-
(a) R. M. Lanigan and T. D. Sheppard, Eur. J. Org. Chem., 2013, 7453–7465 CrossRef CAS
; (b) H. Lundberg, F. Tinnis, N. Selander and H. Adolfsson, Chem. Soc. Rev., 2014, 43, 2714–2742 RSC
; (c) C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405–3415 RSC
; (d) R. García-Álvarez, P. Crochet and V. Cadierno, Green Chem., 2013, 15, 46–66 RSC
; (e) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed
.
-
(a) J. R. Dunetz, Y. Xiang, A. Baldwin and J. Ringling, Org. Lett., 2011, 13, 5048–5051 CrossRef CAS PubMed
; (b) J. D. Goodreid, P. A. Duspara, C. Bosch and R. A. Batey, J. Org. Chem., 2014, 79, 943–954 CrossRef CAS PubMed
; (c) J. Bai, B. K. Zembrón and P. Vogel, Org. Lett., 2014, 16, 604–607 CrossRef CAS PubMed
.
-
(a) P. Starkov and T. D. Sheppard, Org. Biomol. Chem., 2011, 9, 1320–1323 RSC
; (b) R. M. Lanigan, P. Starkov and T. D. Sheppard, J. Org. Chem., 2013, 78, 4512–4523 CrossRef CAS PubMed
; (c) V. Karaluka, R. M. Lanigan, P. M. Murray, M. Badland and T. D. Sheppard, Org. Biomol. Chem., 2015, 13, 10888–10894 RSC
.
-
(a) S. Fatami, N. Gernigon and D. G. Hall, Green Chem., 2015, 17, 4016–4028 RSC
; (b) N. Gernigon, R. M. Al-Zoubi and D. G. Hall, J. Org. Chem., 2012, 77, 8386–8400 CrossRef CAS PubMed
; (c) R. M. Al-Zoubi, O. Marion and D. G. Hall, Angew. Chem., Int. Ed., 2008, 47, 2876–2879 CrossRef CAS PubMed
; (d) H. Lundberg, F. Tinnis and H. Adolfsson, Chem. – Eur. J., 2012, 18, 3822–3826 CrossRef CAS PubMed
; (e) H. Lundberg and H. Adolfsson, ACS Catal., 2015, 5, 3271–3277 CrossRef CAS
; (f) H. Lundberg, F. Tinnis and H. Adolfsson, Synlett, 2012, 2201–2204 CAS
; (g) K. Arnold, B. Davies, R. L. Giles, C. Grosjean, G. E. Smith and A. Whiting, Adv. Synth. Catal., 2006, 348, 813–820 CrossRef CAS
; (h) K. Ishihara, S. Ohara and H. Yamamoto, J. Org. Chem., 1996, 61, 4196–4197 CrossRef CAS PubMed
; (i) C. L. Allen, A. R. Chhatwal and J. M. J. Williams, Chem. Commun., 2012, 48, 666–668 RSC
; (j) K. Arnold, A. S. Batsanov, B. Davies and A. Whiting, Green Chem., 2008, 10, 124–134 RSC
; (k) T. M. El Dine, W. Erb, Y. Berhault, J. Rouden and J. Blanchet, J. Org. Chem., 2015, 80, 4532–4544 CrossRef PubMed
; (l) H. Morimoto, R. Fujiwara, Y. Shimizu, K. Morisaki and T. Ohshima, Org. Lett., 2014, 16, 2018–2021 CrossRef CAS PubMed
; (m) N. Caldwell, C. Jamieson, I. Simpson and A. J. B. Watson, Chem. Commun., 2015, 51, 9495–9498 RSC
; (n) S. Liu, Y. Yang, X. Liu, F. K. Ferdousi, A. S. Batsanov and A. Whiting, Eur. J. Org. Chem., 2013, 5692–5700 CrossRef CAS
; (o) T. M. El Dine, J. Rouden and J. Blanchet, Chem. Commun., 2015, 51, 16084–16087 RSC
.
- The use of a boronic acid catalyst for self-condensation of proline to the corresponding diketopiperazine has been reported, see ref. 8h; Amidation of some protected amino acid derivatives has also been reported, but the scope is limited in most cases: see ref. 8, 9a, k, n and o.
-
T. D. Sheppard, Tris(2,2,2-trifluoroethoxy)boron, ed. P. L. Fuchs, e-EROS Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, 2014
.
- See ESI,† for further details.
- K. Watanabe, N. Yamagiwa and Y. Torisawa, Org. Process Res. Dev., 2007, 11, 251–258 CrossRef CAS
.
- For an eloquent justification for why ‘enantiomeric ratio’ should be employed as a measurement of enantiopurity, see: R. J. Gawley, J. Org. Chem., 2006, 71, 2411–2416 CrossRef CAS PubMed
.
- S. M. Gibson, R. M. Lanigan, L. Benhamou, A. E. Aliev and T. D. Sheppard, Org. Biomol. Chem., 2015, 13, 9050–9054 Search PubMed
.
-
(a) P. Marfey, Carlsberg Res. Commun., 1984, 49, 591–596 CrossRef CAS
; (b) R. Bhushan and H. Brückner, Amino Acids, 2004, 27, 231–247 CrossRef CAS PubMed
.
- W. H. Pirkle, D. L. Sikkenga and M. S. Pavlin, J. Org. Chem., 1977, 42, 384–387 CrossRef CAS
.
- The relatively high degree of racemisation in the amidation of aspartic acid is most likely down to the in situ formation of the cyclic anhydride derivative under the dehydrating conditions.
- A. M. King, C. Salom, J. Dinsmore, E. Salomé-Grosjean, M. De Ryck, R. Kaminski, A. Valade and H. Kohn, J. Med. Chem., 2011, 54, 4815–4830 CrossRef CAS PubMed
.
- Lacosamide is well-known to be prone to racemisation during the amidation/acylation steps in the synthesis:
(a) D. Choi, J. P. Stables and H. Kohn, J. Med. Chem., 1996, 39, 1907–1916 CrossRef CAS PubMed
; (b) P. Morieux, J. P. Stables and H. Kohn, Bioorg. Med. Chem. Lett., 2008, 16, 8968–8975 CrossRef CAS PubMed
; (c) S. Stecko, J. Org. Chem., 2014, 79, 6342–6346 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental procedures and 1H and 13C data for all compounds. See DOI: 10.1039/c6cc05147b |
| ‡ These authors contributed equally. |
| § Current address: Department of Chemistry, Tallinn University of Technology, 15 Akadeemia Rd, 12618 Tallinn, Estonia. |
| This journal is © The Royal Society of Chemistry 2016 |