Open Access Article
Open Access ArticleA biosynthesis-inspired approach to over twenty diverse natural product-like scaffolds†
James D.
Firth
a,
Philip G. E.
Craven
 a,
Matthew
Lilburn
a,
Axel
Pahl
b,
Stephen P.
Marsden
*a and
Adam
Nelson
*ac
a,
Matthew
Lilburn
a,
Axel
Pahl
b,
Stephen P.
Marsden
*a and
Adam
Nelson
*ac
aSchool of Chemistry, University of Leeds, Leeds LS2 9JT, UK. E-mail: a.s.nelson@leeds.ac.uk; s.p.marsden@leeds.ac.uk
bMax Planck Institute of Molecular Physiology, Otto-Hahn Strasse 11, 44227 Dortmund, Germany
cAstbury Centre for Structural Molecular Biology, University of Leeds, Leeds LS2 9JT, UK
First published on 18th July 2016
Abstract
A synthetic approach to diverse scaffolds was developed that was inspired by diterpene biosynthesis. Initial scaffolds, generated using Diels–Alder reactions of furyl-functionalised amines, were transformed into alternative scaffolds using cleavage, ring expansion, annulation and rearrangement reactions. In total, 25 diverse scaffolds were prepared that were shown to have high natural product-likeness.
The exploration of biologically-relevant chemical space is an enduring challenge in both medicinal chemistry and chemical biology. Natural products arise through the evolution of biosynthetic pathways driven by functional benefit to the host organism,1 In biology-oriented synthesis,2 this pre-validated relevance3 is exploited in the design of productive small molecules informed by the frameworks of natural products.4 The structural features of natural products are highly distinctive, and may be captured in a natural product-likeness score.5 In particular, a high fraction of sp3-hybridised carbons6 (Fsp3) is an attractive feature because it correlates strongly with the successful translation of clinical candidates.7 Indeed, about a third of small molecule drugs approved between 1981–2010 were inspired by natural products.8
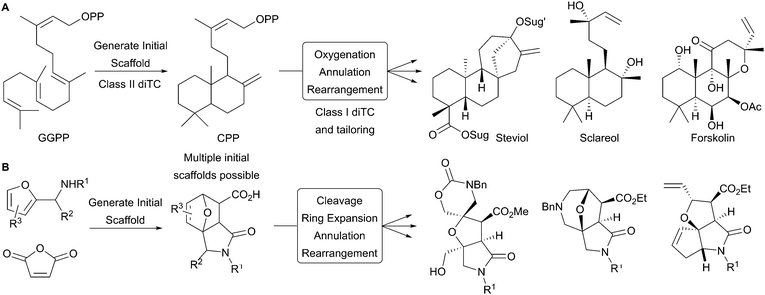
We envisaged a synthetic approach to diverse natural product-like scaffolds that was broadly inspired by diterpene biosynthesis (Scheme 1).9 The bi- and polycyclic scaffolds of labdanes and clerodanes are formed in two steps by diterpene synthases (diTCs). Class II diTCs catalyse the initial cyclisation of geranylgeranyl diphosphate (GGPP) to give bicyclic diphosphate intermediates (Panel A); subsequent enzyme- (e.g. class I diTC) catalysed reactions then yield the final diterpene scaffolds. Inspired by this two-stage synthesis of natural product scaffolds,10 we envisaged that a range of initial scaffolds might be analogously prepared11 by reacting furyl-substituted amines with maleic anhydride (Panel B). The initial scaffolds would then be transformed into product scaffolds using a suite of cleavage, ring expansion, annulation and rearrangement reactions. Thus, analogously to diterpene biosynthesis, sp2-rich starting materials would be transformed into diverse, sp3-rich polycyclic scaffolds.
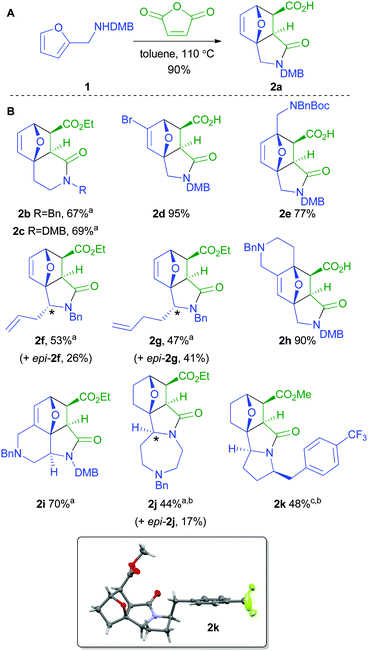
To start with, a range of substituted furyl-substituted amines (ESI†) was reacted with maleic anhydride to give the corresponding cycloadducts 2a–k in moderate to excellent yield (Scheme 2). For example, DMB-protected (2-furyl)-amine 1 yielded the tricyclic adduct 2a in 90% yield as a single diastereomer. Whilst syntheses of similar γ-lactams are known,11 we were delighted that the synthesis of the related12 δ-lactams 2b and 2c was also efficient. Substitution of the 4-11c or 5-11b position of the furan ring was well tolerated (→ 2d and 2e respectively). Substitution α to nitrogen11d,e was also tolerated but the diastereoselectivity was poor: however, after esterification, the diastereomeric cycloadducts (2f/epi-2f and 2g/epi-2f) could be readily separated.
The fusion of addition rings to the furyl-substituted amine starting material was possible in several contexts. For example, the tetracyclic scaffolds 2h and 2i were obtained in 90% and 70% yield respectively (the latter notably as a single diastereomer). In the case of 7-(2-furyl)-1,4-diazepane, esterification and hydrogenation (to prevent retro-cycloaddition) gave the diastereomeric tetracyclic scaffolds 2j and epi-2j in 44% and 17% yield respectively. The reaction of a 2-(2-furyl)-pyrrolidine was, however, more problematic: reaction with maleic anhydride gave an open-chain adduct which underwent (reversible) cycloaddition only after esterification. After subsequent hydrogenation of the thermodynamic mixture of products, the scaffold 2k was obtained in 48% overall yield. The relative configuration of the cycloadducts 2f, epi-2f, epi-2g2j, epi-2j and 2k was determined by X-ray crystallography (Fig. 2 and ESI†).
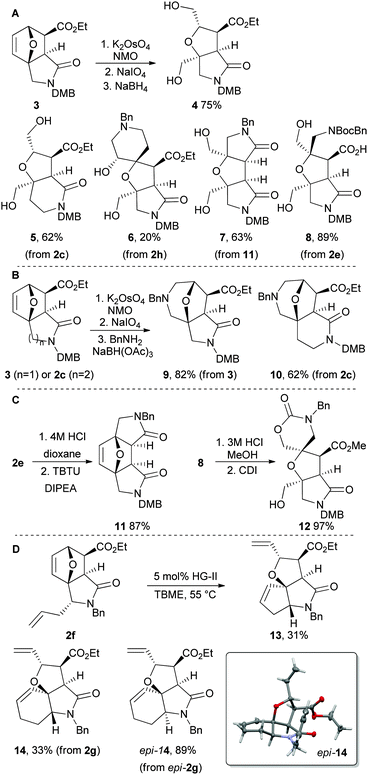
We established a suite of methods to transform the cycloadducts into a wide range of molecular scaffolds (Scheme 3). Initially, we focused on reactions that would cleave (and hence simplify) the initial scaffold (Panel A). Cleavage of the dihydrofuran ring of 3 (from 2a; ESI†) was possible by dihydroxylation of the alkene and treatment with periodate: subsequent reaction with sodium borohydride gave the fused bicyclic scaffold 4 in 75% yield. Crucially, the approach was effective with other initial scaffolds, to give the related bi- and tricyclic scaffolds 5–8; remarkably, the scaffold 6 was obtained as a single diastereomer, albeit in only 20% yield due to a difficult purification.
Next, we focused on formal ring expansions of the initial scaffolds (Panel B).11f After dihydroxylation and oxidative cleavage of 3 and 2c, it was possible to intercept the resulting dialdehydes: thus, treatment with benzylamine and sodium triacetoxyborohydride gave the ring-expanded analogues 9 and 10 in 82% and 62% yield respectively.
Annulation allowed access to more structurally complex scaffolds (Panel C). For example, Boc-deprotection of 2e, followed by TBTU-mediated γ-lactamisation, gave the tetracyclic scaffold 11 in 87% yield. In addition, Boc-deprotection of 8, and concomitant esterification, was followed by treatment with CDI to give the spirocyclic scaffold 12 in 97% yield.
We harnessed alkene metathesis to induce rearrangement of initial scaffolds (Panel D).13 Treatment of the diene 2f with 5 mol% Hoveyda–Grubbs second generation catalyst in TBME gave the tricyclic scaffold 13 in 31% yield. In a similar vein, the dienes 2g and epi-2g were converted into the related tricycles 14 and epi-14. The relative configuration of epi-14 was determined by X-ray crystallography.
Finally, we employed functional group interconversions to modify the substitution of two scaffolds (Scheme 4). These transformations may be considered analogous to steps (e.g. hydroxylation; esterification) that tailor the substitution of diterpene scaffolds. Esterification of 2a, followed by hydrogenation and LiAlH4 reduction, gave the hydroxymethyl-substituted scaffold 15. Mitsunobu reaction of 15 with N-Boc ethyl oxamate (16) as nucleophile, followed by hydrolytic work up,14 gave the orthogonally-protected diamine 17 in 72% yield. Alternatively, transfer hydrogenation of 2a, followed by Curtius rearrangement in the presence of benzyl alcohol, gave the related Cbz-protected scaffold 18 in 67% yield. Finally, esterification of 2d, Suzuki reaction with phenyl boronic acid, and hydrogenation gave the scaffold 19 as a single diastereoisomer (46% yield over 3 steps).
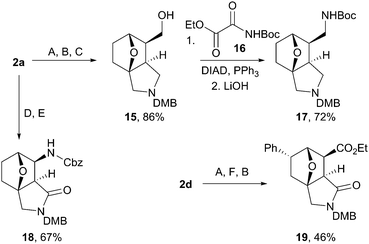 | ||
| Scheme 4 Tailoring of natural product-like scaffolds. Methods: (A) HCl, EtOH; (B) H2, Pd/C; (C) LiAlH4; (D) NH4 HCO2, Pd/C; (E) DPPA, BnOH; (F) PhB(OH)2, K3PO4, 5 mol% Pd(PPh3)4. | ||
In total, 25 scaffolds were prepared using our biosynthesis-inspired approach (ESI†). The scaffolds were highly novel: the Murcko framework15 of only two deprotected scaffolds were substructures in a 2% sample of the ZINC database.16
The diversity of the scaffolds was assessed by constructing a hierarchical scaffold tree (Fig. 1).17 Twenty one different frameworks are represented at the graph-node-bond level, which are related hierarchically to seven parent (monocyclic) frameworks. There is significant scaffold diversity at each level of hierarchy, meaning that the scaffolds are not simply closely related derivatives.
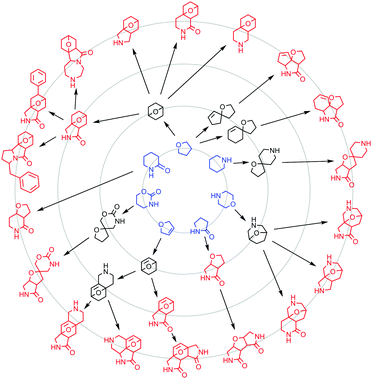 | ||
| Fig. 1 Hierarchical scaffold tree. The twenty one frameworks (red) at the graph-node-bond level are related hierarchically to bicyclic (black) and parent (monocyclic; blue) frameworks. | ||
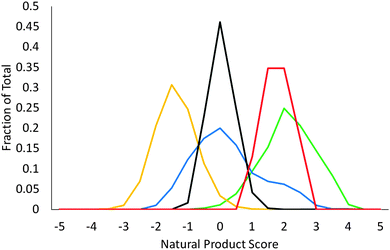
We determined natural product-likeness scores5 for both the 25 (deprotected) scaffolds prepared, and for a range of their derivatives (Fig. 2). A virtual library of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 decorated compounds was enumerated using our open-access tool, LLAMA,18 by combining the 25 (deprotected) scaffolds with up to two typical medicinal chemistry capping groups (ESI†). To assess complementarity to existing collections, natural product-likeness scores were compared with those of 278
223 decorated compounds was enumerated using our open-access tool, LLAMA,18 by combining the 25 (deprotected) scaffolds with up to two typical medicinal chemistry capping groups (ESI†). To assess complementarity to existing collections, natural product-likeness scores were compared with those of 278![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 compounds from a commercial screening collection (μ = −1.63); 1822 FDA-approved drugs (μ = −0.01); and 4460 compounds from a natural product screening library (μ = 1.8) (ESI†). The 25 deprotected scaffolds are highly natural product-like, with a mean natural product-likeness score of 1.58. Even after decoration with typical medicinal chemistry capping groups (μ = −0.21), natural product-likeness is comparable to that of FDA-approved drugs and much higher than that of the commercial screening collection.
365 compounds from a commercial screening collection (μ = −1.63); 1822 FDA-approved drugs (μ = −0.01); and 4460 compounds from a natural product screening library (μ = 1.8) (ESI†). The 25 deprotected scaffolds are highly natural product-like, with a mean natural product-likeness score of 1.58. Even after decoration with typical medicinal chemistry capping groups (μ = −0.21), natural product-likeness is comparable to that of FDA-approved drugs and much higher than that of the commercial screening collection.
The high natural product-likeness of the scaffolds makes them highly attractive for exploitation in the synthesis of distinctive screening compounds. Like diterpenes biosynthesised from GGPP, the scaffolds are much more three-dimensional (e.g. higher Fsp3) than the corresponding starting materials. Crucially, decoration can yield small molecules with good drug-like properties (ESI†). To exploit these favourable properties, >1300 screening compounds have since been prepared from the scaffolds 9, 15 and 17 for addition to the Joint European Compound Library19 (JECL) of the European Lead Factory.
We acknowledge support from the Innovative Medicines Initiative Joint Undertaking under grant number 115489, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (F97/2008-2013), EFPIA companies' in kind contribution for scaffold synthesis and Dr Chris Pask for X-Ray crystallography.
Notes and references
- R. D. Firn and C. G. Jones, Nat. Prod. Rep., 2003, 20, 382 RSC.
- (a) S. Wetzel, R. S. Bon, K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2011, 50, 10800 CrossRef CAS PubMed; (b) H. van Hattum and H. Waldmann, J. Am. Chem. Soc., 2014, 126, 11853 CrossRef PubMed.
- A. L. Harvey, R. Edrada-Ebel and R. J. Quinn, Nat. Rev. Drug Discovery, 2015, 14, 111 CrossRef CAS PubMed.
- For some complementary synthetic approaches to libraries inspired by natural products, see ref. 2 and: (a) D. Morton, S. Leach, C. Cordier, S. Warriner and A. Nelson, Angew. Chem., Int. Ed., 2009, 48, 104 CrossRef CAS PubMed; (b) K. C. Morrison and P. J. Hergenrother, Nat. Prod. Rep., 2014, 31, 6 RSC; (c) A. Masarwa, M. Weber and R. Sarpong, J. Am. Chem. Soc., 2015, 137, 6327 CrossRef CAS PubMed; (d) T. Rodrigues, D. Reker, P. Schneider and G. Schneider, Nat. Chem., 2016, 8, 531 CrossRef CAS PubMed.
- P. Ertl, S. Roggo and A. Schuffenhauer, J. Chem. Inf. Model., 2008, 48, 68 CrossRef CAS PubMed . Natural product likeness scores were calculated using the implementation in RDKit v2015.09.2 (Greg Landrum; Open Source Cheminformatics; http://www.rdkit.org; last accessed 27-Apr-2016).
- (a) H. Lachance, S. Wetzel, K. Kumar and H. Waldmann, J. Med. Chem., 2012, 55, 5989 CrossRef CAS PubMed; (b) D. H. Drewry and R. Macarron, Curr. Opin. Chem. Biol., 2010, 14, 289 CrossRef CAS PubMed.
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2012, 75, 311 CrossRef CAS PubMed.
- (a) P. Zerbe and J. Bohlmann, Trends Biotechnol., 2015, 33, 419 CrossRef CAS PubMed; (b) R. J. Peters, Nat. Prod. Rep., 2010, 27, 1521 RSC; (c) P. M. Dewick, Nat. Prod. Rep., 2002, 19, 181 RSC.
- See also: M. Garcia-Castro, L. Kremer, C. D. Reinkemeier, C. Unkelbach, C. Strohmann, S. Ziegler, C. Ostermann and K. Kumar, Nat. Commun., 2015, 6, 6516 CrossRef CAS PubMed.
- (a) A. V. Varlamov, E. V. Boltukhina, F. I. Zubkov, N. V. Sidorenko, A. I. Chernyshev and D. G. Grudinin, Chem. Heterocycl. Compd., 2004, 40, 22 CrossRef CAS; (b) F. I. Zubkov, V. P. Zaytsev, E. V. Nikitina, V. N. Khrustalev, S. V. Gozun, E. V. Boltukhina and A. V. Varlamov, Tetrahedron, 2011, 67, 9148 CrossRef CAS; (c) R. Murali, H. Surya Prakash Rao and H. W. Scheeren, Tetrahedron, 2001, 57, 3165 CrossRef CAS; (d) E. V. Boltukhina, F. I. Zubkov, E. V. Nikitina and A. V. Varlamov, Synthesis, 2005, 1859 CAS; (e) F. I. Zubkov, E. V. Boltukhina, K. F. Turchin and A. V. Varlamov, Tetrahedron, 2004, 60, 8455 CrossRef CAS; (f) T. Flagstad, G. Min, K. Bonnet, R. Morgentin, D. Roche, M. H. Clausen and T. E. Nielsen, Org. Biomol. Chem., 2016, 14, 4942 RSC.
- (a) R. A. Tromp, J. Brussee and A. van der Gen, Org. Biomol. Chem., 2003, 1, 3592 RSC; (b) M. Treus, L. M. Harwood, J. C. Estévez, C. Salas, M. G. B. Drew and R. J. Estévez, Synlett, 2013, 2221 CAS.
- For related rearrangements, see: (a) J. K. Lam, Y. Schmidt and C. D. Vanderwal, Org. Lett., 2012, 14, 5566 CrossRef CAS PubMed; (b) J. K. Lam, H. V. Pham, K. N. Houk and C. D. Vanderwal, J. Am. Chem. Soc., 2013, 135, 17585 CrossRef CAS PubMed.
- F. Berrée, G. Michelot and M. Le Corre, Tetrahedron Lett., 1998, 39, 8275 CrossRef.
- G. W. Bemis and M. A. Murcko, J. Med. Chem., 1996, 39, 2887 CrossRef CAS PubMed.
- J. J. Irwin, T. Sterling, M. M. Mysinger, E. S. Bolstad and R. G. Coleman, J. Chem. Inf. Model., 2012, 52, 1757 CrossRef CAS PubMed.
- A. Schuffenhauer, P. Ertl, S. Roggo, S. Wetzel, M. A. Koch and H. Waldmann, J. Chem. Inf. Model., 2007, 47, 47 CrossRef CAS PubMed.
- I. Colomer, C. J. Empson, P. Craven, Z. Owen, R. G. Doveston, I. Churcher, S. P. Marsden and A. Nelson, Chem. Commun., 2016, 52, 7209 RSC ; LLAMA is available at http://https://llama.leeds.ac.uk.
- (a) J. Besnard, P. S. Jones, A. L. Hopkins and A. D. Pannifer, Drug Discovery Today, 2015, 20, 181 CrossRef CAS PubMed; (b) A. Karawajczyk, F. Giordanetto, J. Benningshof, D. Hamza, T. Kalliokoski, K. Pouwer, R. Morgentin, A. Nelson, G. Müller, A. Piechot and D. Tzalis, Drug Discovery Today, 2015, 20, 1310 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data, computational analyses and crystallographic data in CIF or other electronic forms. CCDC 1478957–1478963. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc04662b |
| This journal is © The Royal Society of Chemistry 2016 |