Open Access Article
Open Access ArticleSilver migration between Au38(SC2H4Ph)24 and doped AgxAu38−x(SC2H4Ph)24 nanoclusters†
Bei
Zhang
,
Giovanni
Salassa
and
Thomas
Bürgi
*
Department of Physical Chemistry, University of Geneva, 30 Quai Ernest-Ansermet, 1211 Geneva 4, Switzerland. E-mail: thomas.buergi@unige.ch
First published on 17th June 2016
Abstract
A fast redistribution of metal atoms occurs upon mixing the AgxAu38−x and Au38 nanoclusters in solution, as observed by mass spectrometry. Physical separation of AgxAu38−x and Au38 species by a dialysis membrane prohibits the metal migration, which suggests that collisions between the reacting clusters are at the origin of the observation.
Thiolate protected gold nanoclusters have gained increasing interest due to their precise atomic composition.1 The structure of the well-defined Au nanoclusters comprises a gold metallic core and protecting SR-(Au–SR)n staple units. The Au38(SC2H4Ph)24 nanocluster, for example, is composed of a Au23 core, six dimeric staple motifs (SR–Au–SR–Au–SR) and three monomeric staples (SR–Au–SR).2 The mobility of the thiolates on the cluster surface was evidenced by racemization of the Au38 nanocluster enantiomer, which may involve a series of inter-staple SN2-type reactions.3 In addition, Negishi4 and Murray5 both reported an intercluster ligand exchange between gold nanoclusters, suggesting the detachment of ligands or gold–ligand complexes. This is in agreement with older studies on self-assembled monolayers of alkylthiolates on flat gold surfaces, which revealed desorption of radiolabeled thiols into the solution.6 From recent results in the literature, Au and doped Au clusters seem to be very dynamic systems that are able to easily re-organize themselves.7,8
Heteroatoms have been introduced in the core and staples of gold nanoclusters to impart enhanced chemical and physical properties.9–17 Doping increased the nanocluster surface flexibility and therefore lowered the racemization temperature of chiral Au38.18,19 One of the recently studied methods to obtain bimetallic and trimetallic gold nanoclusters is metal exchange between hetero-metal thiolates and well defined gold nanoclusters.20,21 Pradeep and coworkers reported the synthesis of AgAu nanoclusters by instantaneous intercluster metal exchange between Ag44(SR)30 and Au25(SR)18 nanoclusters.22 However, no experimental study has been carried out to address the intercluster metal exchange mechanism. There are two possible pathways: (1) small metal thiolate species (metal A) detach from one nanocluster and attack the other nanocluster (metal B); (2) the two nanocluster exchange metal atoms upon collision.
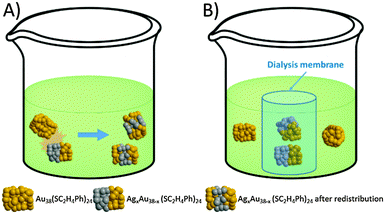
Here we report the migration of silver atoms between AgxAu38−x and Au38 nanoclusters, which was observed by MALDI mass spectrometry. In order to investigate the metal migration mechanism, a dialysis membrane was applied to separate AgxAu38−x and Au38 nanoclusters spatially (Scheme 1), while allowing the transfer of small species across the membrane. No metal migration occurs under these conditions, pointing towards a collision mechanism for the reaction.
Three batches (denoted A, B and C) of the AgxAu38−x nanocluster were synthesized by metal exchange between the Au38 nanocluster and AgSC2H4Ph (for details see the ESI†). All the three batches showed very similar Ag distribution with species from x = 3 to x = 11 (“x” being the number of Ag atoms in the cluster). It should be noted that fresh samples were used in this study and multiple passages over a size exclusion column were performed to avoid interference from small species. In the preliminary test, the AgxAu38−x nanocluster (batch A) was mixed with Au38 in a mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
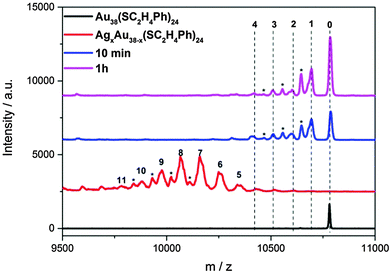
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Upon mixing with Au38, the green solution of AgxAu38−x turned brown. Fig. 1 shows the MALDI spectra of starting AgxAu38−x and the mixture after 10 minutes and after 1 hour. After 10 minutes, the mixture showed new AgxAu38−x species with x around 1 and 2. Meanwhile the starting AgxAu38−x clusters with x > 5 have disappeared. We calculated the average number of dopant atoms as:
1. Upon mixing with Au38, the green solution of AgxAu38−x turned brown. Fig. 1 shows the MALDI spectra of starting AgxAu38−x and the mixture after 10 minutes and after 1 hour. After 10 minutes, the mixture showed new AgxAu38−x species with x around 1 and 2. Meanwhile the starting AgxAu38−x clusters with x > 5 have disappeared. We calculated the average number of dopant atoms as:
![[x with combining macron]](https://www.rsc.org/images/entities/b_char_0078_0304.gif) = ∑xi × Ai/∑Ai = ∑xi × Ai/∑Ai |
![[x with combining macron]](https://www.rsc.org/images/entities/b_char_0078_0304.gif) in the cluster passed from 7.6 to 1.2 only in 10 minutes. The surprisingly low average number of silver atoms after mixing could be due to more pronounced fragmentation of AgxAu38−x with a larger x. No significant changes are noted from MALDI spectra after 1 h mixing, indicating that most of the reaction takes place within the first ten minutes.
in the cluster passed from 7.6 to 1.2 only in 10 minutes. The surprisingly low average number of silver atoms after mixing could be due to more pronounced fragmentation of AgxAu38−x with a larger x. No significant changes are noted from MALDI spectra after 1 h mixing, indicating that most of the reaction takes place within the first ten minutes.
The UV-vis spectra of Au38 and AgxAu38−x are clearly different (Fig. S2a, ESI,† compare black and red spectra). In an attempt to follow the evolution of the reaction, UV-vis spectra were collected immediately after the mixing of AgxAu38−x (batch B) with Au38 and every minute for the following 10 minutes, then one after 20 minutes and 30 minutes. These spectra are reported in Fig. S2b (ESI†). No clear changes were observed in the spectra recorded at different times after the mixing. Difference spectra obtained by subtracting the first spectrum recorded after mixing from all the subsequent spectra (Fig. S2c, ESI†) showed only minor changes. It turned out that the UV-vis spectrum of the product AgxAu38−x (with an intermediate ![[x with combining macron]](https://www.rsc.org/images/entities/b_char_0078_0304.gif) ) could be synthesized by the superposition of the spectra of the original AgxAu38−x cluster (with large
) could be synthesized by the superposition of the spectra of the original AgxAu38−x cluster (with large ![[x with combining macron]](https://www.rsc.org/images/entities/b_char_0078_0304.gif) ) and Au38 (see the fit in the Fig. S3, ESI†). This means that the absorption spectroscopy is not very sensitive to the migration of metal atoms in this case.
) and Au38 (see the fit in the Fig. S3, ESI†). This means that the absorption spectroscopy is not very sensitive to the migration of metal atoms in this case.
AgxAu38−x (batch C, average dopant number ![[x with combining macron]](https://www.rsc.org/images/entities/b_char_0078_0304.gif) = 6.0) was mixed with Au38 in two mass ratios (AgxAu38−x
= 6.0) was mixed with Au38 in two mass ratios (AgxAu38−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au38 = 2
Au38 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The MALDI spectrum (Fig. S4, ESI†) shows that in both cases the distribution of AgxAu38−x clusters is shifted to lower x values after mixing with Au38. As expected the mixture containing more silver has a distribution with higher x values.
1). The MALDI spectrum (Fig. S4, ESI†) shows that in both cases the distribution of AgxAu38−x clusters is shifted to lower x values after mixing with Au38. As expected the mixture containing more silver has a distribution with higher x values.
We furthermore compared the MALDI spectrum of batch B immediately after synthesis and size exclusion and after stirring the sample for three hours. As shown in Fig. S5 (ESI†) the average number of silver atoms remained centred around x = 6.5 but the distribution of species with different x became sharper with time. This indicates that metal exchange takes place between AgxAu38−x clusters as well. The observed change in compositional distribution reflects a thermodynamic driving force towards a narrower distribution. The narrow distribution indicates that silver incorporation is thermodynamically favoured in specific positions in the Au38 cluster, as was evidenced recently by Dass and coworkers.10 In the latter's work it was shown that silver preferentially occupies nine positions on the cluster core surface. In fact the distribution of species AgxAu38−x observed after three hours of stirring (Fig. S5, ESI†) is close to a binomial distribution of 6.4 Ag atoms over nine possible sites (see Fig. S6, ESI†). The same number of silver atoms distributed over 38 sites gives a much broader binomial distribution. This indicates that the system evolves towards a situation where the silver atoms occupy a restricted number of sites in the cluster.
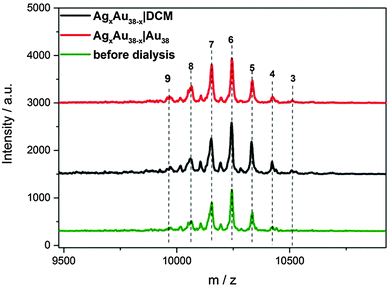
To study the mechanism of this fast Ag migration from AgxAu38−x to Au38, experiments were conducted using a dialysis membrane. This allows one to physically separate the two clusters, whereas the transport of small species (<1000 Da) through the membrane is possible. To check the performance of the dialysis membrane a DCM solution of 2-phenylethanethiol (2-PET) was placed inside the membrane and pure DCM was placed outside (the dialysis reaction is denoted as 2-PET|DCM, the same format is applied for the following dialysis study). After dialysis, the outside DCM solution showed the characteristic peaks of 2-PET in 1H-NMR (Fig. S7, ESI†). The same condition was applied for AgxAu38−x|DCM and AgxAu38−x|Au38 (AgxAu38−x batch B). After dialysis, the AgxAu38−x inside the membrane showed no changes in the distribution of Ag and Au (Fig. 2). Note that in the AgxAu38−x|DCM experiment no clusters were found outside the membrane, showing that the clusters are too large to penetrate the pores of the membrane.
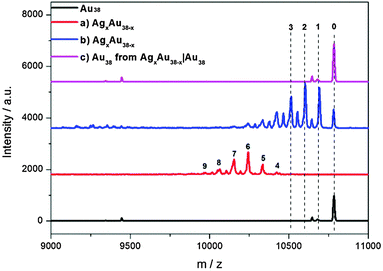
In the AgxAu38−x|Au38 dialysis study, no exchanged species were found in the outside solution (Fig. 3). The concentrated outside solution did not show silver migration products (x > 0) after 3 h (Fig. S8, ESI†). In the reference experiment using the same AgxAu38−x and Au38 solutions but without dialysis membrane migration is proved by MALDI (Fig. 3). These findings show that the mechanism of Ag migration does not involve small species that detach from the AgxAu38−x cluster and react with Au38. This suggests a mechanism of metal atom exchange involving collisions between the reacting clusters. In our previous report19 we showed, using racemization studies, that AgxAu38−x has a significantly higher surface flexibility compared to Au38 due to the weaker Ag–S bond compared to Au–S. The high flexibility of the AgxAu38−x surface may play an important role in the collision mechanism for the metal atom migration observed here. It was proposed earlier3 that the observed racemization of Au38 could take place via inter-staple SN2-type reactions whereby the staples on the surface of the cluster are mixed among each other. Possibly, metal migration occurs during collision in a similar fashion between clusters therefore mixing staples belonging to different clusters.
In conclusion, silver migration was observed between AgxAu38−x and Au38 upon mixing in solution. This reaction seems to proceed very fast, on the timescale of minutes, at least. The silver migration kinetics could be further studied by fluorescence experiments. The distribution of AgxAu38−x species with different composition (different x) in solution depends on the starting AgxAu38−x and Au38 ratio. However, the migration was not detected when the two clusters were physically separated by a dialysis membrane, which allowed small species to diffuse across the membrane. This suggests a mechanism for the metal migration that involves collisions between the reacting clusters. The high flexibility of the AgxAu38−x cluster surface, as observed before, may play an important factor in this mechanism.
This work was supported by The Swiss National Science Foundation (grant number 200020_152596). B. Z. thanks The China Scholarship Council (No. 201306340012).
Notes and references
- H. Häkkinen, Nat. Chem., 2012, 4, 443–455 CrossRef PubMed.
- H. Qian, W. T. Eckenhoff, Y. Zhu and R. Jin, J. Am. Chem. Soc., 2010, 25, 8280–8281 CrossRef PubMed.
- S. Knoppe, I. Dolamic and T. Burgi, J. Am. Chem. Soc., 2012, 134, 13114–13120 CrossRef CAS PubMed.
- Y. Niihori, W. Kurashige, M. Matsuzaki and Y. Negishi, Nanoscale, 2013, 5, 508–512 RSC.
- Y. Song, T. Huang and R. W. Murray, J. Am. Chem. Soc., 2003, 125, 11694–11701 CrossRef CAS PubMed.
- J. B. Schlenoff, M. Li and H. Ly, J. Am. Chem. Soc., 1995, 117, 12528–12536 CrossRef CAS.
- T. Bürgi, Nanoscale, 2015, 7, 15553–15567 RSC.
- W. Kurashige, Y. Niihori, S. Sharma and Y. Negishi, J. Phys. Chem. Lett., 2014, 5, 4134–4142 CrossRef CAS PubMed.
- R. C. Jin and K. Nobusada, Nano Res., 2014, 7, 285–300 CrossRef CAS.
- C. Kumara, K. J. Gagnon and A. Dass, J. Phys. Chem. Lett., 2015, 6, 1223–1228 CrossRef CAS PubMed.
- B. Zhang, S. Kaziz, H. Li, D. Wodka, S. Malola, O. Safonova, M. Nachtegaal, C. Mazet, I. Dolamic, J. Llorca, E. Kalenius, L. M. Lawson Daku, H. Hakkinen, T. Bürgi and N. Barrabés, Nanoscale, 2015, 7, 17012–17019 RSC.
- B. Molina and A. Tlahuice-Flores, Phys. Chem. Chem. Phys., 2016, 18, 1397–1403 RSC.
- T. Cesca, B. Kalinic, N. Michieli, C. Maurizio, A. Trapananti, C. Scian, G. Battaglin, P. Mazzoldi and G. Mattei, Phys. Chem. Chem. Phys., 2015, 17, 28262–28269 RSC.
- F. Muniz-Miranda, M. C. Menziani and A. Pedone, J. Phys. Chem. C, 2015, 119, 10766–10775 CrossRef CAS.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS PubMed.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. Abdulhalim, M. R. Parida, A. H. Emwas, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 5749–5753 CrossRef CAS PubMed.
- M. Zhou, J. Zhong, S. X. Wang, Q. J. Guo, M. Z. Zhu, Y. Pei and A. D. Xia, J. Phys. Chem. C, 2015, 119, 18790–18797 CrossRef CAS.
- N. Barrabes, B. Zhang and T. Burgi, J. Am. Chem. Soc., 2014, 136, 14361–14364 CrossRef CAS PubMed.
- B. Zhang and T. Burgi, J. Phys. Chem. C, 2016, 120, 4660–4666 CrossRef CAS.
- S. X. Wang, Y. B. Song, S. Jin, X. Liu, J. Zhang, Y. Pei, X. M. Meng, M. Chen, P. Li and M. Z. Zhu, J. Am. Chem. Soc., 2015, 137, 4018–4021 CrossRef CAS PubMed.
- S. Yang, S. Wang, S. Jin, S. Chen, H. Sheng and M. Zhu, Nanoscale, 2015, 7, 10005–10007 RSC.
- K. R. Krishnadas, A. Ghosh, A. Baksi, I. Chakraborty, G. Natarajan and T. Pradeep, J. Am. Chem. Soc., 2016, 138, 140–148 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, dialysis experiment, MALDI, UV-vis, and 1HNMR measurement results described in the text. See DOI: 10.1039/c6cc04469g |
| This journal is © The Royal Society of Chemistry 2016 |