Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvent responsive catalyst improves NMR sensitivity via efficient magnetisation transfer†
Amy J.
Ruddlesden
 and
Simon B.
Duckett
and
Simon B.
Duckett
 *
*
Centre for Hyperpolarisation in Magnetic Resonance (CHyM), York Science Park, University of York, Heslington, York, YO10 5NY, UK. E-mail: simon.duckett@york.ac.uk; Tel: +44 (0)1904 322564
First published on 2nd June 2016
Abstract
A bidentate iridium carbene complex, Ir(κC,O-L1)(COD), has been synthesised which contains a strongly electron donating carbene ligand that is functionalised by a cis-spanning phenolate group. This complex acts as a precursor to effective magnetisation transfer catalysts which form after reaction with H2 and a suitable two electron donor. In solvents such as benzene, containing pyridine, they are exemplified by neutral, chiral Ir(H)2(κC,O-L1)(py)2 with inequivalent hydride ligands and Ir–O bond retention, whilst in methanol, Ir–O bond cleavage leads to zwitterionic [Ir(H)2(κC,O−-L1)(py)3]+, with chemically equivalent hydride ligands. The active catalyst's form is therefore solvent dependent. Both these complexes break the magnetic symmetry of the hydride ligands and are active in the catalytic transfer of polarisation from parahydrogen to a loosely bound ligand. Test results on pyridine, nicotinaldehyde and nicotine reveal up to ≈1.2% single spin proton polarisation levels in their 1H NMR signals which compare to the normal 0.003% level at 9.4 Tesla. These results exemplify how rational catalyst design yields a solvent dependent catalyst with good SABRE activity.
The use of hyperpolarisation methods to overcome the inherent insensitivity of NMR spectroscopy reflects an area of research where Parahydrogen Induced Polarisation (PHIP) features heavily.1 The incorporation of parahydrogen (p-H2), a nuclear singlet, into a molecule was first shown to produce an enhanced NMR signal in 1987.2 The increase in signal intensity for resonances arising from, or coupled to, p-H2 derived protons, has since been the subject of intense investigation. Recently a p-H2 technique that does not chemically change a molecule, called Signal Amplification By Reversible Exchange (SABRE), has been developed.3 Polarisation is transferred through J-coupling between the p-H2 derived hydride ligands and the substrate ligands.3 Exchange with free substrate and fresh p-H2 enables the build-up of polarisation in the substrate pool through multiple visits to the catalyst. The most commonly used catalysts are cationic species which contain either phosphine4,5 or N-heterocyclic carbene (NHC) ligands.6,7
In fact, one of the most effective magnetisation transfer catalysts is Ir(COD)(IMes)Cl7 [where IMes = 1,3-bis(2,4,6-trimethylphenyl)imidazole-2-ylidene, COD = cyclooctadiene] which forms the charged complex, [Ir(H)2(IMes)(substrate)3]Cl once activated with H2 and a substrate. This SABRE catalyst contains chemically equivalent but magnetically inequivalent hydride ligands and polarisation transfer has proven particularly efficient in polar protic solvents such as methanol. Furthermore, using this catalyst a wide range of substrates including nicotinamide,8–10 isoniazid11 and pyrazole12 have been shown to become hyperpolarised. This type of approach has been exemplified for 1H, 13C, 31P, 19F and 15N nuclei.5,13–15 However, due to the charged nature of such a species, it has proven less efficient in the range of low polarity solvents commonly used in NMR analysis.
It has also been shown that species with chemically inequivalent hydride ligands can act as SABRE catalysts. One reported example of this class of catalyst is given by [Ir(H)2(CH3CN)(IMes)(PCy3)(pyridine)]Cl.16 Recently, a system that exhibits a wider solvent tolerance has been developed. It contains a bidentate ligand which binds through carbene and phenolate centres, resulting in a neutral catalyst in all solvents tested. While it was found to exhibit greater activity in non-polar solvents such as benzene,17 it was not efficient in methanol due to much slower ligand exchange.
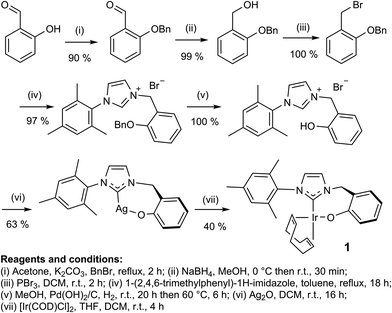
In this study, the related neutral iridium complex, Ir(κC,O-L1)(COD), 1, [where L1 = 3-(2-methylene-phenolate)-1-(2,4,6-trimethylphenyl)imidazolylidene], has been synthesised starting from salicylaldehyde. It contains a cis-spanning phenolate-substituted bidentate NHC (see Scheme 1). Benzyl protection of the phenol18 allowed conversion of the aldehyde to the bromide19via the alcohol.20 Addition of 1-(2,4,6-trimethylphenyl)-1H-imidazole then formed the imidazolium bromide salt.21 Deprotection followed by silver carbene formation and subsequent transmetallation22 afforded the product, the phenoxide iridium carbene complex, 1. Key compounds have been characterised by NMR and MS as illustrated in the ESI.†
In solution, complex 1 appears yellow/orange in colour. UV-Vis analysis in DCM showed it exhibits three absorption bands in the visible region of the spectrum (373, 425 and 490 nm) with absorption coefficients in the range of charge transfer transitions (1017, 1326 and 249 dm3 mol−1 cm−1). Work by Perutz et al.23–26 has assigned similar bands in related square planar metal complexes to metal d–p transitions although earlier assignments suggested they were metal-to-ligand charge transfer bands.27–29
At room temperature, the 1H NMR spectrum of complex 1 in CD2Cl2 yields well resolved resonances (see Fig. 1). Two doublets at δ 6.53 and 5.17 are observed for the CH2 linker protons which have a common 2J(HH) coupling of 14.9 Hz. These two protons are diastereotopic due to the seven-membered metallocycle which is indicative of the retention of the Ir–O bond.30 Complex 1 is air/moisture sensitive but stable as a solid and in solution under N2. It also forms stable complexes once fully reacted with substrate and hydrogen as detailed in the following reactions.
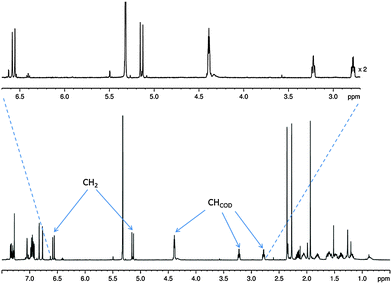 | ||
| Fig. 1 1H NMR spectrum of 1 showing evidence for the diastereotopic CH2 linker protons with key CH resonances labelled. | ||
Upon cooling a CD2Cl2 solution of complex 1 to 243 K all of its NMR signals become broad and undefined due to fluxional behaviour and when pyridine is added to this solution no reaction is evident. If, however, H2 gas is added at 255 K, a limited reaction occurs as two pairs of hydride signals are now seen at δ −12.35 and −18.25 (1.7% conversion) and at δ −12.39 and −17.64 (0.6%). These minor hydride containing products are conformational isomers of compound 2 (see Scheme 3) which arise due to differing metallocycle orientations;30 analogous behaviour has been reported.17
When p-H2 is used in this reaction, these hydride ligand signals do not show any significant 1H NMR signal enhancement. However, the free H2 signal in these spectra is substantial which suggests that 1 undergoes rapid and reversible oxidative addition of H2 to form 2 which consumes the p-H2. There is no indication of the hydrogenation of COD at 255 K and upon warming to 298 K, these hydride signals broaden into the baseline of the corresponding NMR spectra and strong signals for 1 are always visible.
When a CD2Cl2 solution of 1 reacts with both pyridine and H2 at 298 K a new neutral iridium species, Ir(H)2(κC,O-L1)(py)2, 3 is observed to slowly form containing two bound pyridine environments in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (see ESI† for details). This species is also formed in C6D6. The mutually coupled inequivalent hydride signals appear in CD2Cl2 at δ −22.55 and −25.49 (2J(HH) = 8.1 Hz) and in C6D6 at δ −21.94 and −24.52 (2J(HH) = 7.7 Hz). Their inequivalence suggests Ir–O bond retention, a fact which is further supported by the diastereotopic nature of the CH2 linker protons which appear as doublets in both CD2Cl2 and C6D6. Both complexes undergo pyridine and H2 exchange as highlighted in Scheme 2, although only the pyridine ligand trans to hydride dissociates. The use of EXSY NMR31 experiments enabled determination of experimental rate constants for pyridine loss in 3 at 294 K of 3.74 ± 0.06 s−1 in CD2Cl2 and 13.5 ± 0.6 s−1 in C6D6. The corresponding H2 loss rates were 0.80 ± 0.01 s−1 and 3.02 ± 0.07 s−1 respectively and are therefore much slower than those of pyridine loss.
1 (see ESI† for details). This species is also formed in C6D6. The mutually coupled inequivalent hydride signals appear in CD2Cl2 at δ −22.55 and −25.49 (2J(HH) = 8.1 Hz) and in C6D6 at δ −21.94 and −24.52 (2J(HH) = 7.7 Hz). Their inequivalence suggests Ir–O bond retention, a fact which is further supported by the diastereotopic nature of the CH2 linker protons which appear as doublets in both CD2Cl2 and C6D6. Both complexes undergo pyridine and H2 exchange as highlighted in Scheme 2, although only the pyridine ligand trans to hydride dissociates. The use of EXSY NMR31 experiments enabled determination of experimental rate constants for pyridine loss in 3 at 294 K of 3.74 ± 0.06 s−1 in CD2Cl2 and 13.5 ± 0.6 s−1 in C6D6. The corresponding H2 loss rates were 0.80 ± 0.01 s−1 and 3.02 ± 0.07 s−1 respectively and are therefore much slower than those of pyridine loss.
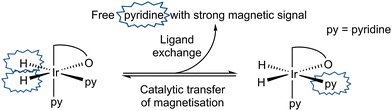 | ||
| Scheme 2 Transfer of hydride polarisation into the indicated pyridine ligand is followed by ligand exchange to build-up hyperpolarised pyridine in solution. | ||
This behaviour changes significantly upon moving to protic methanol. Now, the addition of H2 to a CD3OD solution of complex 1 at 250 K, results in a very limited reaction to form a dihydride (<1%, with resonances at δ −12.65 and −18.27 and the low concentration presumably prevents observation of its conformational isomer) but upon warming further, rapid and total decomposition of 1 follows. In contrast, the addition of pyridine to a CD3OD solution of complex 1 at 243 K forms the phenolate dissociation product, square planar 4 quantitatively (see Scheme 3 and ESI†) where the COD ligand yields four inequivalent alkene proton resonances.
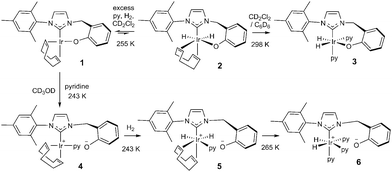 | ||
| Scheme 3 Species formed during the reaction of 1 with H2 and pyridine in CD3OD (4, 5 and 6) and CD2Cl2 (2 and 3) or C6D6 (3) solution. | ||
Upon the addition of p-H2 and pyridine to 4 at 243 K, two PHIP enhanced hydride signals become immediately visible at δ −12.34 and −17.50 that are shown to couple via COSY. They arise from H2 addition to 4 which initially forms the alkene dihydride complex, 5. The CH2 linker protons of 5 remain diastereotopic on phenolate rotation due to the absence of a mirror plane. Upon warming to 265 K, this system evolves further and a single hydride signal becomes visible at δ −22.18 due to the formation of [Ir(H)2(κC,O−-L1)(py)3]+, 6; the COD is converted to cyclooctene. Further NMR analysis confirms that 6 contains two bound pyridine ligand environments, in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, two hydride ligands and an iridium–carbene bond. Its CH2 linker protons are now equivalent, appearing as a singlet at δ 4.83 due to the existence of a mirror plane as detailed in Scheme 3.
1 ratio, two hydride ligands and an iridium–carbene bond. Its CH2 linker protons are now equivalent, appearing as a singlet at δ 4.83 due to the existence of a mirror plane as detailed in Scheme 3.
Complex 6 is zwitterionic, and its cationic Ir(III) centre is balanced by phenoxide ion formation. Hence the final reaction product formed from 1 with pyridine and H2 is solvent dependent. The two pyridine ligands of 6 that lie trans to hydride are shown to dissociate with an experimental rate constant of 1.35 ± 0.03 s−1 at 294 K, although no exchange of the pyridine trans to the carbene is observed. In CD3OD, rapid H/D exchange, accompanied by HD formation, is observed which prevents the quantification of the H2 loss rate in this solvent. At 294 K, the ligand exchange rates of species 3 in C6D6 are therefore much faster than those in CD2Cl2, but both are faster than those of 6 in CD3OD as shown in the ESI.†
The ligand exchange rates of 6 in CD3OH were examined as a function of temperature and activation parameters for these processes were calculated (see ESI†). The activation enthalpy values for both pyridine and hydride loss are very similar to each other (90.7 ± 1.6 kJ mol−1 and 88.3 ± 9.1 kJ mol−1 respectively). The entropy of activation values of 71.3 ± 5.3 J K−1 and 56.1 ± 30.6 J K−1 for pyridine and hydride loss respectively are also similar and positive thereby confirming the dissociative character of these steps.7 Similar ligand exchange processes, in a series of related complexes, yield values of similar size.7,32 A ligand exchange mechanism featuring reversible pyridine dissociation with H2 loss via [Ir(κC,O−-L1)(H)2(H2)(py)2]+ is therefore indicated.33
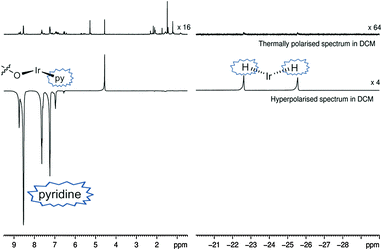
Both catalysts 3 and 6 therefore exhibit the substrate and H2 exchange characteristics required for them to act as SABRE catalysts. To test their substrate signal enhancing performance, samples were prepared containing catalyst 1 and a chosen substrate in the desired NMR solvent. These were rigorously degassed before the addition of 3 bars of H2. Samples were tested by reintroducing p-H2 into the headspace of the NMR tube, shaking the sample in the low field outside of the spectrometer and then rapidly transferring the sample into the spectrometer for examination. This resulted in the observation of enhanced signals in the corresponding single scan 1H NMR spectra for the hydride ligands, bound substrate and free substrate in solution as exemplified by Fig. 2. The total enhancement value seen for the five protons of pyridine in C6D6 proved to be 1850-fold under the conditions detailed. For CD2Cl2 this enhancement level was reduced to ca. 1660-fold whilst for CD3OD solution it became 710-fold. Given the corresponding gain in signal-to-noise levels that these enhancements provide, the resulting time savings are substantial in all solvents. For comparison purposes, the site specific enhancement values for the substrates pyridine, nicotinaldehyde and nicotine are summarised in Table 1. The performance of 1 as a SABRE catalyst for pyridine like substrates has therefore been established.
| Solvent | Pyridine 1H NMR SABRE enhancement (fold) | ||
|---|---|---|---|
| ortho | meta | para | |
| C6D6 | 843 ± 27 | 600 ± 30 | 404 ± 13 |
| CD2Cl2 | 877 ± 32 | 337 ± 57 | 450 ± 22 |
| CD3OD | 426 ± 5 | 47 ± 28 | 243 ± 3 |
| Solvent | Nicotinaldehyde 1H NMR SABRE enhancement (fold) | |||
|---|---|---|---|---|
| HA | HB | HC | HD | |
| C6D6 | 259 ± 17 | 209 ± 22 | 118 ± 22 | 244 ± 18 |
| CD2Cl2 | 486 ± 36 | 400 ± 32 | 148 ± 34 | 396 ± 65 |
| CD3OD | 62 ± 7 | 59 ± 10 | 26 ± 7 | 65 ± 6 |
| Solvent | Nicotine 1H NMR SABRE enhancement (fold) | |||
|---|---|---|---|---|
| HA | HB | HC | HD | |
| C6D6 | 400 ± 54 | 315 ± 48 | 88 ± 30 | 338 ± 80 |
| CD2Cl2 | 419 ± 51 | 381 ± 48 | 146 ± 44 | 386 ± 55 |
| CD3OD | 29 ± 4 | 28 ± 3 | 3 ± 3 | 31 ± 3 |
It is known that the amount of signal enhancement observed in the 1H NMR spectrum of a substrate under SABRE is significantly affected by the ligand exchange rates, as previously explained by Green et al.34 For comparison, [Ir(H)2(IMes)(py)3]Cl, is commonly used as the catalyst benchmark for SABRE and its pyridine dissociation rate32 was found to be 23 s−1. While the ligand dissociation rates for 3 and 6 are much lower, they still achieve good hyperpolarisation levels. In fact, these data show that one pyridine substrate trans to hydride in CD2Cl2 or C6D6 is more efficient at receiving SABRE than the two equivalent pyridine ligands of 6 in CD3OD. Furthermore, the concept of an active solvent responsive catalyst is illustrated.
In summary, the iridium precatalyst Ir(κC,O-L1)(COD), 1 containing a phenolate substituted NHC has been synthesised and shown to act as an efficient SABRE catalyst precursor. The active catalytic species is solvent dependent. Complex 1 contains an Ir–O bond which is affected by solvent polarity and proton availability; in non-polar C6D6 and polar aprotic CD2Cl2, this bond is strong and substitution resistant with 1 forming Ir(κC,O-L1)(H)2(py)2 (3) on reaction with H2 and pyridine. In contrast, on changing to polar protic methanol, the Ir–O bond becomes labile and the phenolate easily dissociates from the iridium centre, such that zwitterionic [Ir(κC,O−-L1)(H)2(py)3]+ (6) forms. 6 is directly analogous to the efficient SABRE catalyst [Ir(H)2(IMes)(py)3]Cl which performs well in CD3OD but has lower activity in non-polar CD2Cl2 and C6D6. Both 3 and 6 undergo pyridine and H2 exchange thereby enabling them to act as SABRE catalysts. Whilst 6 works well in CD3OD, catalyst neutrality in the non-polar solvents CD2Cl2 and C6D6 results in the formation of 3 which is highly active for SABRE catalysis. This study therefore shows that catalyst design and control can lead to improved magnetisation transfer in a range of solvents, a requirement for future studies that seek to identify low concentration analytes35–37 and to produce hyperpolarised MRI contrast agents.
We acknowledge Bruker Biospin and the EPSRC for a studentship (1359398) and the Wellcome Trust and Wolfson Foundation (092506 and 098335) for their generous funding. We thank Dr Jason Lynam, Prof. Gary Green and Dr Ryan Mewis for helpful comments.
References
- T. C. Eisenschmid, R. U. Kirss, P. P. Deutsch, S. I. Hommeltoft, R. Eisenberg, J. Bargon, R. G. Lawler and A. L. Balch, J. Am. Chem. Soc., 1987, 109, 8089 CrossRef CAS.
- C. R. Bowers and D. P. Weitekamp, J. Am. Chem. Soc., 1987, 109, 5541 CrossRef CAS.
- R. W. Adams, S. B. Duckett, R. A. Green, D. C. Williamson and G. G. R. Green, J. Chem. Phys., 2009, 131, 194505 CrossRef PubMed.
- K. D. Atkinson, M. J. Cowley, S. B. Duckett, P. I. P. Elliott, G. G. R. Green, J. Lopez-Serrano, I. G. Khazal and A. C. Whitwood, Inorg. Chem., 2009, 48, 663 CrossRef CAS PubMed.
- K. D. Atkinson, M. J. Cowley, P. I. P. Elliott, S. B. Duckett, G. G. R. Green, J. Lopez-Serrano and A. C. Whitwood, J. Am. Chem. Soc., 2009, 131, 13362 CrossRef CAS PubMed.
- B. J. A. van Weerdenburg, S. Gloggler, N. Eshuis, A. H. J. Engwerda, J. M. M. Smits, R. de Gelder, S. Appelt, S. S. Wymenga, M. Tessari, M. C. Feiters, B. Blumich and F. P. J. T. Rutjes, Chem. Commun., 2013, 49, 7388 RSC.
- M. J. Cowley, R. W. Adams, K. D. Atkinson, M. C. R. Cockett, S. B. Duckett, G. G. R. Green, J. A. B. Lohman, R. Kerssebaum, D. Kilgour and R. E. Mewis, J. Am. Chem. Soc., 2011, 133, 6134 CrossRef CAS PubMed.
- J.-B. Hoevener, N. Schwaderlapp, R. Borowiak, T. Lickert, S. B. Duckett, R. E. Mewis, R. W. Adams, M. J. Burns, L. A. R. Highton, G. G. R. Green, A. Olaru, J. Hennig and D. von Elverfeldtt, Anal. Chem., 2014, 86, 1767 CrossRef CAS PubMed.
- R. E. Mewis, K. D. Atkinson, M. J. Cowley, S. B. Duckett, G. G. R. Green, R. A. Green, L. A. R. Highton, D. Kilgour, L. S. Lloyd, J. A. B. Lohman and D. C. Williamson, Magn. Reson. Chem., 2014, 52, 358 CrossRef CAS PubMed.
- M. L. Truong, F. Shi, P. He, B. Yuan, K. N. Plunkett, A. M. Coffey, R. V. Shchepin, D. A. Barskiy, K. V. Kovtunov, I. V. Koptyug, K. W. Waddell, B. M. Goodson and E. Y. Chekmenev, J. Phys. Chem. B, 2014, 118, 13882 CrossRef CAS PubMed.
- H. Zeng, J. Xu, J. Gillen, M. T. McMahon, D. Artemov, J.-M. Tyburn, J. A. B. Lohman, R. E. Mewis, K. D. Atkinson, G. G. R. Green, S. B. Duckett and P. C. M. van Zijl, J. Magn. Reson., 2013, 237, 73 CrossRef CAS PubMed.
- E. B. Dücker, L. T. Kuhn, K. Münnemann and C. Griesinger, J. Magn. Reson., 2012, 214, 159 CrossRef PubMed.
- A. N. Pravdivtsev, A. V. Yurkovskaya, H. Zimmermann, H.-M. Vieth and K. L. Ivanov, RSC Adv., 2015, 5, 63615 RSC.
- L. T. Kuhn, U. Bommerich and J. Bargon, J. Phys. Chem. A, 2006, 110, 3521 CrossRef CAS PubMed.
- V. V. Zhivonitko, I. V. Skovpin and I. V. Koptyug, Chem. Commun., 2015, 51, 2506 RSC.
- M. Fekete, O. Bayfield, S. B. Duckett, S. Hart, R. E. Mewis, N. Pridmore, P. J. Rayner and A. Whitwood, Inorg. Chem., 2013, 52, 13453 CrossRef CAS PubMed.
- A. J. Ruddlesden, R. E. Mewis, G. G. R. Green, A. C. Whitwood and S. B. Duckett, Organometallics, 2015, 34, 2997 CrossRef CAS PubMed.
- V. L. Schuster, Y. Chi, A. S. Wasmuth and R. S. Pottorf, G. L. Olson and W. I. P. Organisation, WO2011/37610 A1, Albert Einstein College Of Medicine Of Yeshiva University, US, 2011 Search PubMed.
- G. Sagrera and G. Seoane, Synthesis, 2009, 4190 CAS.
- R. V. Somu, H. Boshoff, C. Qiao, E. M. Bennett, C. E. Barry and C. C. Aldrich, J. Med. Chem., 2005, 49, 31 CrossRef PubMed.
- G. Occhipinti, V. R. Jensen, K. W. Tornroos, N. A. Froystein and H. R. Bjorsvik, Tetrahedron, 2009, 65, 7186 CrossRef CAS.
- J. L. Herde, J. C. Lambert and C. V. Senoff and M. A. Cushing, Inorg. Syn., John Wiley & Sons, Inc., 2007 Search PubMed.
- M. V. Campian, R. N. Perutz, B. Procacci, R. J. Thatcher, O. Torres and A. C. Whitwood, J. Am. Chem. Soc., 2012, 134, 3480 CrossRef CAS PubMed.
- M. C. Nicasio, R. N. Perutz and A. Tekkaya, Organometallics, 1998, 17, 5557 CrossRef CAS.
- L. Cronin, M. C. Nicasio, R. N. Perutz, R. G. Peters, D. M. Roddick and M. K. Whittlesey, J. Am. Chem. Soc., 1995, 117, 10047 CrossRef CAS.
- C. Hall, W. D. Jones, R. J. Mawby, R. Osman, R. N. Perutz and M. K. Whittlesey, J. Am. Chem. Soc., 1992, 114, 7425 CrossRef CAS.
- G. L. Geoffroy, H. Isci, J. Litrenti and W. R. Mason, Inorg. Chem., 1977, 16, 1950 CrossRef CAS.
- R. Brady, B. R. Flynn, G. L. Geoffroy, H. B. Gray, J. Peone and L. Vaska, Inorg. Chem., 1976, 15, 1485 CrossRef CAS.
- G. L. Geoffroy, G. S. Hammond and H. B. Gray, J. Am. Chem. Soc., 1975, 97, 3933 CrossRef CAS.
- H. M. Peng, R. D. Webster and X. Li, Organometallics, 2008, 27, 4484 CrossRef CAS.
- K. Stott, J. Keeler, Q. N. Van and A. J. Shaka, J. Magn. Reson., 1997, 125, 302 CrossRef CAS.
- L. S. Lloyd, A. Asghar, M. J. Burns, A. Charlton, S. Coombes, M. J. Cowley, G. J. Dear, S. B. Duckett, G. R. Genov, G. G. R. Green, L. A. R. Highton, A. J. J. Hooper, M. Khan, I. G. Khazal, R. J. Lewis, R. E. Mewis, A. D. Roberts and A. J. Ruddlesden, Catal. Sci. Technol., 2014, 4, 3544 Search PubMed.
- D. A. Barskiy, A. N. Pravdivtsev, K. L. Ivanov, K. V. Kovtunov and I. V. Koptyug, Phys. Chem. Chem. Phys., 2016, 18, 89 RSC.
- R. A. Green, R. W. Adams, S. B. Duckett, R. E. Mewis, D. C. Williamson and G. G. R. Green, Prog. Nucl. Magn. Reson. Spectrosc., 2012, 67, 1 CrossRef CAS PubMed.
- N. Eshuis, N. Hermkens, B. J. A. van Weerdenburg, M. C. Feiters, F. P. J. T. Rutjes, S. S. Wijmenga and M. Tessari, J. Am. Chem. Soc., 2014, 136, 2695 CrossRef CAS PubMed.
- N. Eshuis, R. L. E. G. Aspers, B. J. A. van Weerdenburg, M. C. Feiters, F. P. J. T. Rutjes, S. S. Wijmenga and M. Tessari, Angew. Chem., Int. Ed., 2015, 54, 14527 CrossRef CAS PubMed.
- N. Eshuis, B. J. A. van Weerdenburg, M. C. Feiters, F. P. J. T. Rutjes, S. S. Wijmenga and M. Tessari, Angew. Chem., Int. Ed., 2015, 54, 1481 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; synthesis and characterisation of compounds, SABRE experiments, kinetic data, activation parameters, UV-vis data. The underlying research data for this paper is available in accordance with EPSRC open data policy from DOI: 10.15124/cdaf969f-ba47-401e-876b-512c0cece55c. See DOI: 10.1039/c6cc03185d |
| This journal is © The Royal Society of Chemistry 2016 |