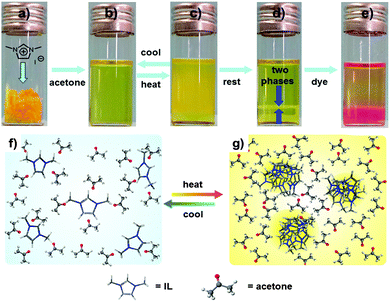
Open Access Article
Open Access ArticleLower critical solution temperature (LCST) phase behaviour of an ionic liquid and its control by supramolecular host–guest interactions†
Shengyi
Dong
a,
Jan
Heyda
*b,
Jiayin
Yuan
c and
Christoph A.
Schalley
*a
aInstitut für Chemie und Biochemie, Freie Universität Berlin, Takustrasse 3, 14195 Berlin, Germany. E-mail: c.schalley@fu-berlin.de
bPhysical Chemistry Department, University of Chemistry and Technology, Prague Technicka 5, 16628 Prague 6, Czech Republic. E-mail: jan.heyda@vscht.cz
cMax Planck Institute of Colloids and Interfaces, 14476 Potsdam, Germany
First published on 26th May 2016
Abstract
Lower critical solution temperature (LCST) phase behaviour of an imidazolium-based ionic liquid is reported, which can be controlled by concentration, the choice of cation, anion and solvent, and by supramolecular host–guest complex formation. Molecular dynamics simulations provide insight into the molecular basis of this LCST phenomenon. This thermo-responsive system has potential applications in cloud point extraction processes.
The implementation of controllable and programmable stimuli-responsiveness into materials is pivotal for smart function and the realization of applications.1 Lower critical solution temperature (LCST) phase behaviour in solution is an intensely studied property of a series of thermo-responsive materials. They are miscible with solvents – in most cases water – only below a critical temperature.2 So far, the investigation of LCST phase behaviour overwhelmingly focused on covalent polymers.2 Classic examples such as poly(N-isopropylacrylamide) and its derivatives have many applications in the areas of nano- and biotechnology.2c,m Comparably, much less attention has so far been paid to low molecular weight compounds exhibiting LCST behaviour, although such phenomena may have practical values for example in extraction processes.
Ionic liquids (ILs) are organic salts with melting points below 100 °C and offer unique physicochemical properties.3 They have gained importance for diverse areas such as green chemistry, supramolecular chemistry and catalysis.3 ILs are not only tunable solvents to promote the thermo-responsive behaviour of the solutes, but some of them display LCST-type phase transition in water or organic solvents themselves.4 Though ILs with LCST phase transition behaviour have been reported, attention is mainly focused on the phase transition phenomena,4 while a mechanistic understanding of this behaviour at the molecular level still needs to be developed.2h,4,5 Furthermore, the parameters to fine-tune the LCST behaviour of ILs have been limited to concentration and solvent so far – a quite severe restriction for the implementation of applications. More options to tailor thermo-responsiveness would thus be a great advantage for function.2,6
Here, we report the LCST phase behaviour of an ionic liquid, 1,3-dimethylimidazolium iodide, in acetone combining experiment and molecular dynamics (MD) simulations in order to get more detailed insight into the molecular basis of the phenomenon. As an additional tuning option, we introduce supramolecular control of the thermal behaviour and demonstrate that different hosts for the ionic liquid offer control over the phase transition temperature.
At room temperature, 1,3-dimethylimidazolium iodide (IL-I) is soluble in acetone and forms a transparent, light-yellow solution. When heated to 50 °C, the acetone solution of IL-I turns turbid first and then separates into an IL-I phase at the bottom of the vial and an acetone phase immiscible with it on top (Fig. 1). After cooling down the IL-I/acetone phase-separated mixture to room temperature, a single transparent phase forms again (Fig. 1). This temperature-induced mixing–demixing is thus fully reversible and has not been described for IL-I/acetone so far. In contrast, no such LCST behaviour was observed for IL-I in other solvents such as water, methanol, ethanol, acetonitrile, DMF and DMSO (Fig. S1 and S2, ESI†). These observations indicate that the LCST-type phase transition of IL-I is a unique, solvent-specific phenomenon in acetone.4a
In order to obtain detailed insight into this LCST phase behaviour, cloud points were determined by light transmittance measurements at a wavelength (λ) of 550 nm for differently concentrated IL-I/acetone mixtures at various temperatures (Fig. 2a). At IL-I concentrations of 300, 400, and 500 mg mL−1, the corresponding cloud point temperatures (Tcloud) defined as 50% of the initial transmittance at λ = 550 nm are 49.4, 46.5 and 44.8 °C, respectively. All transitions are sharp, typically within 1 °C from the beginning to the end of the transitions. At even higher IL-I concentrations (>600 mg mL−1), a reversed Tcloud/concentration relationship is observed. The Tcloud/concentration phase diagram (Fig. 2b) exhibits a V-shaped curve separating the single-phase from the double-phase region. Such a V-shaped Tcloud curve is a typical feature of LCST-type ionic liquids and also typical for most polymer-based LCST systems.
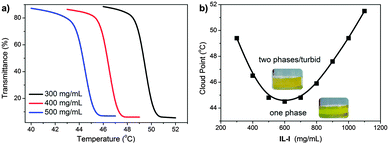 | ||
| Fig. 2 (a) Temperature-dependence of light transmittance of mixtures of IL-I and acetone. (b) Concentration dependence of cloud point of IL-I in acetone. | ||
As ionic liquids are salts, the nature of the ions plays a crucial role for their solubility as well as their thermo-responsiveness.4g Therefore, other counterions and a more lipophilic cation were examined. When I− in IL-I was replaced by Cl−, PF6− or n-C8H17OSO3−, respectively, or when 1-pentyl-3-methylimidazolium iodide was used, no LCST phase separations occurred. These results demonstrate both the cation and anion in IL-I to be important for thermo-responsiveness, i.e. the LCST behaviour of IL-I is structure-specific and depends on both the ion–solvent and anion–cation interactions.
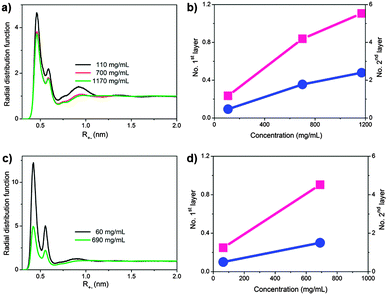
All atom molecular dynamics (MD) simulations were performed to unravel the demixing mechanism at the molecular level and gain a more profound understanding into the microscopic processes occurring in IL/acetone mixtures (Fig. 3 and Fig. S4–S8, ESI†).7 More detailed insight into the role of the anions (Cl−vs. I−), IL concentration, and temperature were gained, as the simulations allowed measuring cation–anion affinity in terms of radial distribution functions. IL-I is more or less homogeneously dissolved in acetone at room temperature and the interactions among individual IL-I ion pairs are strengthened at higher temperatures, resulting in the aggregation of IL-I, the release of solvating acetone molecules into the bulk and, as a consequence, the observed LCST behaviour (Fig. S4, ESI†). Furthermore, the concentration of the ionic liquid has only a modest effect on the solution structure (the height of contact ion-pair peak decreases from 4.5 at 110 mg mL−1 only to 3.8 at 1170 mg mL−1, Fig. S3a and S4, ESI†).
The same simulation protocol was applied to IL-Cl (1,3-dimethylimidazolium chloride).7 As shown in Fig. 3c and Fig. S5 (ESI†), in the diluted regime, the chloride anion has a strong affinity to 1,3-dimethylimidazolium (at any temperature), which can be attributed to its smaller size and its therefore ‘harder’ character. For solution thermodynamics of IL-Cl, an obvious difference to the iodide salt exists: the ionic liquid–ionic liquid affinity decreases with growing temperatures, indicating that IL-Cl cannot form large-scale aggregates. The simulations thus agree with experiment in that no LCST behaviour of IL-Cl is observed in acetone and support the finding that the LCST phenomenon is sensitive to changes in the chemical nature of the ionic liquid involved.
The probabilities of contact ion pair formation at different concentrations were simulated and also gave us a clear comparison between different anions (Fig. 3a and c). The cation–Cl− affinity is very strong at low concentration; however, it rapidly decreases when the concentration increases. In marked contrast, the cation–I− affinity is virtually concentration-independent, having clear consequences for the aggregating and clustering abilities. In the case of IL-I, larger clusters (or mesoscopic, dense IL-I regions) form and lead to the clouding and subsequent demixing, while for IL-Cl, the cation–anion affinity substantially decreases with increased concentration, making the IL-Cl effectively increasingly miscible with acetone at higher IL-Cl concentrations thus prohibiting phase separation.
Imidazolium-based ILs have been reported to form host–guest complexes with macrocyclic hosts, including pillararenes such as P5 and crown ethers like DB24C8 (chemical structures in Scheme 1).8 Previous work demonstrated the important role of supramolecular interactions inducing LCST behaviour.6a The introduction of supramolecular interactions in the LCST system under study here provides us with an additional tool to control the thermo-responsiveness beyond mere concentration, solvent and counterion changes. The addition of increasing amounts of P5 leads to a clear decrease of Tcloud (Fig. 4). In the case of DB24C8, the opposite trend is observed. Upon increasing the concentration of DB24C8 (IL-I: 500 mg mL−1), Tcloud increases correspondingly. Supramolecular interactions can consequently be used to control the thermo-responsiveness6a even in significantly different ways.
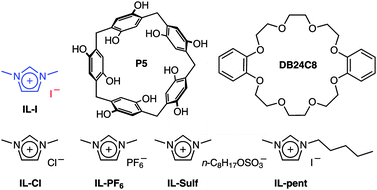 | ||
| Scheme 1 Chemical structures of ionic liquids, and two macrocyclic compounds (pillar[5]arene P5 and crown ether DB24C8) which are known hosts for the imidazolium cation. | ||
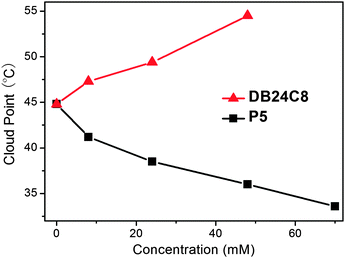 | ||
| Fig. 4 Supramolecular control over the LCST-type phase behaviour through different macrocyclic compounds. The concentration of IL-I for each measurement is at 500 mg mL−1, 2.2 M. | ||
At present, we can only speculate, why the two complexes behave differently. With DB24C8, complexes with the ionic liquid form, which are more soluble and decompose at higher temperature. The higher the concentration of the crown, the higher the concentration of complexes and the lower the concentration of native ionic liquid. Consequently, the cloud point temperatures increase with higher crown ether concentration as the dissociation of most of the complexes is required to generate enough free ionic liquid to induce the LCST transition. This mechanism does not easily rationalize the opposite trend observed for the pillararene. Here, two effects likely counterbalance each other. One is the host–guest complex formation as discussed for the crown ether. The second one is likely an effect on the solvent properties caused by the presence of many OH groups on the pillararene. At least, other OH-rich molecules such as different carbohydrates are also known to cause a downwards trend of cloud point temperatures in polymeric LCST materials.9 If this second effect over-compensates the host–guest effect, decreasing cloud point temperatures are expected, when the concentration of the host is increased. The host or the complex or both are thus directly involved in the LCST transition rather than being merely a modifying agent for the amount of ionic liquid.
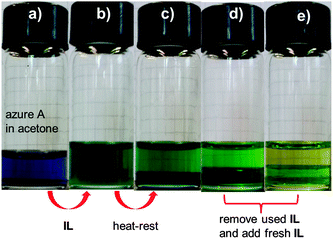
Acetone is a common solvent in organic chemistry and the reversible thermo-responsive mixing and demixing processes in acetone might be useful to realize cloud point extraction (CPE) as a separation procedure (Fig. 5 and Fig. S3, ESI†). An organic dye, azure A, which is soluble in acetone, was applied as the organic model molecule. After the addition of azure A to acetone, the whole acetone solution becomes purple. When IL-I is added to this solution, the color turns green due to the mixing of the purple acetone and the yellowish IL-I phases at room temperature. Upon heating and resting, demixing leads to two immiscible layers, with the major fraction of the dye residing in the IL-I layer. After removal of dye-containing IL-I layer, fresh IL-I is added and the extraction repeated. After three cycles, virtually all the azure A is extracted from the acetone layer. These observations indicate that this IL-I-based LCST system is useful for applications in mixture separation and purification.4a,6a,9
In conclusion, a rather unique LCST phase behaviour of an imidazolium-based ionic liquid in acetone has been described. This LCST phase behaviour can be conveniently controlled by changing concentration, cation, anion and solvent. Supramolecular interactions between the ionic liquid and two macrocyclic hosts control the phase transition temperatures – surprisingly with opposite concentration dependences of the cloud temperatures. Molecular dynamics simulations are consistent with the observed thermo-responsiveness, and provide insight into the molecular basis of the observed thermo-responsive behaviour as well as the role of the counterions. Our very simple thermo-responsive system has potential for applications in separation processes. Considering the easy commercial availability of both acetone and IL-I, this binary system is thus of practical importance.
S. D. was supported with a postdoctoral fellowship by the Alexander von Humboldt Foundation.
Notes and references
-
(a) F. Huang and H. W. Gibson, Prog. Polym. Sci., 2005, 30, 982 CrossRef CAS
; (b) J. L. Sessler, D. E. Gross, W.-S. Cho, V. M. Lynch, F. P. Schmidtchen, G. W. Bates, M. E. Light and P. A. Gale, J. Am. Chem. Soc., 2006, 128, 12281 CrossRef CAS PubMed
; (c) Z. Niu and H. W. Gibson, Chem. Rev., 2009, 109, 6024 CrossRef CAS PubMed
; (d) Y. Zhou, W. Huang, J. Liu, X. Zhu and D. Yan, Adv. Mater., 2010, 22, 4567 CrossRef CAS PubMed
; (e) G. J. E. Davidson, S. Sharma and S. J. Loeb, Angew. Chem., Int. Ed., 2010, 49, 4938 CrossRef CAS PubMed
; (f) J. Wu, A. Zawistowski, M. Ehrmann, T. Yi and C. Schmuck, J. Am. Chem. Soc., 2011, 133, 9720 CrossRef CAS PubMed
; (g) S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905 CrossRef CAS PubMed
; (h) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC
; (i) B. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D'Souza, A. E. Fernandes and D. A. Leigh, Science, 2013, 339, 189 CrossRef CAS PubMed
; (j) Y. Liu, Z. Wang and X. Zhang, Chem. Soc. Rev., 2012, 41, 5922 RSC
; (k) Y. Hisamatsu, S. Banerjee, M. B. Avinash, T. Govindaraju and C. Schmuck, Angew. Chem., Int. Ed., 2013, 52, 12550 CrossRef CAS PubMed
; (l) G. L. Fiore, S. J. Rowan and C. Weder, Chem. Soc. Rev., 2013, 42, 7278 RSC
; (m) X. Yan, J.-F. Xu, T. R. Cook, F. Huang, Q.-Z. Yang, C.-H. Tung and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 8717 CrossRef CAS PubMed
; (n) M. Zhang, X. Yan, F. Huang, Z. Niu and H. W. Gibson, Acc. Chem. Res., 2014, 47, 1995 CrossRef CAS PubMed
; (o) C. J. Bruns and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 2186 CrossRef CAS PubMed
; (p) D. Asil, J. A. Foster, A. Patra, X. de Hatten, J. del Barrio, O. A. Scherman, J. R. Nitschke and R. H. Friend, Angew. Chem., Int. Ed., 2014, 53, 8388 CrossRef CAS PubMed
.
-
(a) J. Lachwa, J. Szydlowski, V. N. Visak, L. P. N. Rebelo, K. R. Seddon, M. N. Ponte, J. M. S. S. Esperança and H. J. R. Guedes, J. Am. Chem. Soc., 2005, 127, 6542 CrossRef CAS PubMed
; (b) S. Schmitz and H. Ritter, Angew. Chem., Int. Ed., 2005, 44, 5658 CrossRef CAS PubMed
; (c) J.-F. Lutz, Ö. Akdemir and A. Hoth, J. Am. Chem. Soc., 2006, 128, 13046 CrossRef CAS PubMed
; (d) H. Zhang and A. I. Cooper, Adv. Mater., 2007, 19, 2439 CrossRef CAS
; (e) M. Chiper, D. Fournier, R. Hoogenboom and U. S. Schubert, Macromol. Rapid Commun., 2008, 29, 1640 CrossRef CAS
; (f) T. Hirose, M. Irie and K. Matsuda, Adv. Mater., 2008, 20, 2137 CrossRef CAS
; (g) S. Amajjahe and H. Ritter, Macromolecules, 2008, 41, 3250 CrossRef CAS
; (h) J. E. Betancourt and J. M. Rivera, J. Am. Chem. Soc., 2009, 131, 16666 CrossRef CAS PubMed
; (i) M. I. Gibson, D. Paripovic and H.-A. Klok, Adv. Mater., 2010, 22, 4721 CrossRef CAS PubMed
; (j) J.-F. Lutz, Adv. Mater., 2011, 23, 2237 CrossRef CAS
; (k) T. Sun and G. Qing, Adv. Mater., 2011, 23, H57 CrossRef CAS PubMed
; (l) G. Ju, M. Cheng, M. Xiao, J. Xu, K. Pan, X. Wang, Y. Zhang and F. Shi, Adv. Mater., 2013, 25, 2915 CrossRef CAS PubMed
; (m) Z.-X. Zhang, K. L. Liu and J. Li, Angew. Chem., Int. Ed., 2013, 52, 6180 CrossRef CAS PubMed
.
-
(a) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed
; (b) M. J. Earle, J. M. S. S. Esperança, M. A. Gilea, J. N. C. Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831 CrossRef CAS PubMed
; (c) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC
; (d) Y. Yang, A. J. Mijalis, H. Fu, C. Agosto, K. J. Tan, J. D. Batteas and D. E. Bergbreiter, J. Am. Chem. Soc., 2012, 134, 7378 CrossRef CAS PubMed
; (e) J. Yuan, D. Mecerreyes and M. Antonietti, Prog. Polym. Sci., 2013, 38, 1009 CrossRef CAS
; (f) N. Nishimura and H. Ohno, Polymer, 2014, 55, 3289 CrossRef CAS
.
-
(a) K. Fukumoto and H. Ohno, Angew. Chem., Int. Ed., 2007, 46, 1852 CrossRef CAS PubMed
; (b) Y. Kohno and H. Ohno, Phys. Chem. Chem. Phys., 2012, 14, 5063 RSC
; (c) M. L. S. Batista, L. I. N. Tomé, C. M. S. S. Neves, E. M. Rocha, J. R. B. Gomest and J. A. P. Coutinho, J. Phys. Chem. B, 2012, 116, 5985 CrossRef CAS PubMed
; (d) T. Ando, Y. Kohno, N. Nakamura and H. Ohno, Chem. Commun., 2013, 49, 10248 RSC
; (e) Y. Men, H. Schlaad and J. Yuan, ACS Macro Lett., 2013, 2, 456 CrossRef CAS
; (f) S. Montolio, L. Gonzáez, B. Altava, H. Tenhu, M. I. Burguete, E. G. Verdugo and S. V. Luis, Chem. Commun., 2014, 50, 10683 RSC
; (g) M. Smiglak, J. M. Pringle, X. Lu, L. Han, S. Zhang, H. Gao, D. R. MacFarlane and R. D. Rogers, Chem. Commun., 2014, 50, 9228 RSC
; (h) Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Polym. Chem., 2015, 6, 2163 RSC
.
-
(a) F. Wang, A. Klaikherd and S. Thayumanavan, J. Am. Chem. Soc., 2011, 133, 13496 CrossRef CAS PubMed
; (b) S. Lee, J.-S. Lee, C. H. Lee, Y.-S. Jun and J.-M. Kim, Langmuir, 2011, 27, 1560 CrossRef CAS PubMed
; (c) S. Sata, Y. Mieno, Y. Kohno and H. Ohno, Chem. Commun., 2014, 50, 15450 RSC
.
-
(a) S. Dong, B. Zheng, Y. Yao, C. Han, J. Yuan, M. Antonietti and F. Huang, Adv. Mater., 2013, 25, 6864 CrossRef CAS PubMed
; (b) A. Das and S. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 1092 CrossRef CAS PubMed
; (c) X. Chi, X. Ji, D. Xia and F. Huang, J. Am. Chem. Soc., 2015, 137, 1440 CrossRef CAS PubMed
.
-
(a) M. Parrinello and A. Rahman, J. Appl. Phys., 1981, 52, 7182 CrossRef CAS
; (b) J. N. Canongia Lopes and A. A. H. Pádua, J. Phys. Chem. B, 2006, 110, 19586 CrossRef CAS PubMed
; (c) J. Schmidt, C. Krekeler, F. Dommert, Y. Zhao, R. Berger, L. D. Site and C. Holm, J. Phys. Chem. B, 2010, 114, 6150 CrossRef CAS PubMed
; (d) J. Heyda, M. Lund, M. Ončák, P. Slavíček and P. Jungwirth, J. Phys. Chem. B, 2010, 114, 10843 CrossRef CAS PubMed
; (e) C. Caleman, P. J. van Maaren, M. Hong, J. S. Hub, L. T. Costa and D. van der Spoel, J. Chem. Theory Comput., 2012, 8, 61 CrossRef CAS PubMed
; (f) M. Reinhardt, J. Dzubiella, M. Trapp, P. Gutfreund, M. Kreuzer, A. H. Gröschel, A. H. E. Müller, M. Ballauff and R. Steitz, Macromolecules, 2013, 46, 6541 CrossRef CAS
.
-
(a) D. Castillo, P. Astudillo, J. Mares, F. J. González, A. Vela and J. Tiburcio, Org. Biomol. Chem., 2007, 5, 2252 RSC
; (b) C. Li, L. Zhao, J. Li, X. Ding, S. Chen, Q. Zhang, Y. Yu and X. Jia, Chem. Commun., 2010, 46, 9016 RSC
; (c) M. Lee, Z. Niu, D. V. Schoonover, C. Slebodnick and H. W. Gibson, Tetrahedron, 2010, 66, 7077 CrossRef CAS
; (d) K. Zhu, V. N. Vukotic and S. J. Loeb, Angew. Chem., Int. Ed., 2012, 51, 2168 CrossRef CAS PubMed
; (e) S. I. M-Olivares, R. Cervantes and J. Tiburcio, J. Org. Chem., 2013, 78, 10724 CrossRef PubMed
; (f) L. Gao, Y. Yao, S. Dong and J. Yuan, RSC Adv., 2014, 4, 35489 RSC
; (g) S. Dong, J. Yuan and F. Huang, Chem. Sci., 2014, 5, 247 RSC
.
- See for example:
(a) A. Sjöberg, G. Karlström and F. Tjerneld, Macromolecules, 1989, 22, 4512–4516 CrossRef
; (b) B. Y. Zaslavsky, Two-phase Partitioning – Physical Chemistry and Bioanalytical Applications, CRC Press, Boca Raton, 1994 Search PubMed
. Also, ions are known to cause complex LCST behaviour in polymeric systems: (c) S. Sasaki and S. Okabe, J. Phys. Chem. B, 2011, 115, 12905–12910 CrossRef CAS PubMed
; (d) J. Heyda and J. Dzubiella, J. Phys. Chem. B, 2014, 118, 10979–10988 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, cloud temperature determination, calixarene control experiments. See DOI: 10.1039/c6cc02838a |
| This journal is © The Royal Society of Chemistry 2016 |