Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface-directed modulation of supramolecular gel properties†
Maria Galini Faidra
Angelerou
a,
Akmal
Sabri
a,
Rhiannon
Creasey
 b,
Polyxeni
Angelerou
c,
Maria
Marlow
*a and
Mischa
Zelzer
*ad
b,
Polyxeni
Angelerou
c,
Maria
Marlow
*a and
Mischa
Zelzer
*ad
aUniversity of Nottingham, School of Pharmacy, Nottingham NG7 2RD, UK. E-mail: pazmem@exmail.nottingham.ac.uk; pazmz@exmail.nottingham.ac.uk
bSchool of Chemistry, University of Queensland, St Lucia, 4067, Australia
cDelft University of Technology, School of Civil Engineering, 2628 CD, Delft, Netherlands
dNational Physical Laboratory, Teddington, Middlesex TW11 0LW, UK
First published on 22nd February 2016
Abstract
Supramolecular materials are widely studied and used for a variety of applications; in most applications, these materials are in contact with surfaces of other materials. Whilst much focus has been placed on elucidating factors that affect supramolecular material properties, the influence of the material surface on gel formation is poorly characterised. Here, we demonstrate that surface properties directly affect the fibre architecture and mechanical properties of self-assembled cytidine based gel films.
Supramolecular materials are under intense investigation in many areas such as conducting materials, energy and information storage, tissue engineering, sensors, coatings or catalysis1–3 due to their ability to self-heal, mimic biological functionalities and form structures with precise nano-scale order and interactions.4 The functionality and application potential of supramolecular materials is closely linked to their chemical, physical and mechanical properties which in turn are affected by processing conditions and gelation triggers such as concentration, pH, temperature, solvent and enzymes.5–7
Among the factors affecting supramolecular self-assembly, the influence of the surrounding material surface on bulk gel properties has received little attention. The effect of surfaces on the formation of self-assembled monolayers is well established8 and a recent example demonstrated that the structure of a surface (i.e. graphite) can provide a template for monolayer formation and guide 2D self-assembly of molecules (p-terphenyl-3,5,3′′,5′′-tetracarboxylic acid) with matching dimensions.9
As surfaces can template and influence the self-assembly of monolayers we hypothesised that material surfaces may also have the potential to influence self-assembly of gelators and hence affect the properties of the resulting gels. To date the interplay between surface properties and bulk gelation is poorly understood and existing reports mostly focus on confinement of the self-assembly trigger to the surface. For example, it was shown that self-assembly can be triggered with surface immobilised enzymes to form fibres10 or electrochemically to form gel films.11 A direct influence of surface morphology on self-assembly was observed when diphenylalanine was placed in contact with either glass or a microporous mixed cellulose ester membrane, where the gelator formed nanofibres and microvesicles, respectively.7,12
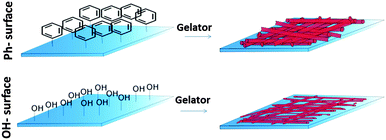
Although the material surface has been recognised to play an important role in the self-assembly process13 the effect of this interaction on gel properties has not yet been demonstrated. Here, we report for the first time a direct relation between material surface properties and the physical properties of a gel film formed on the surface (Fig. 1).
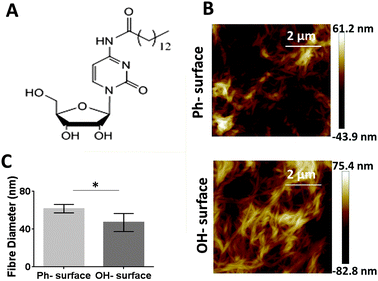
To investigate the effect of surface properties on physical gel characteristics we formed gel films from one recently synthesized cytidine derivative, the N-(1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)octanamide (C14-cytidine)14 on surfaces displaying two different chemical functionalities. The gelator is currently under investigation as a class of drug delivery system with low toxicity14 and we expect that its amphiphilic nature would enable it to interact differently with surfaces of high or low hydrophobicity.
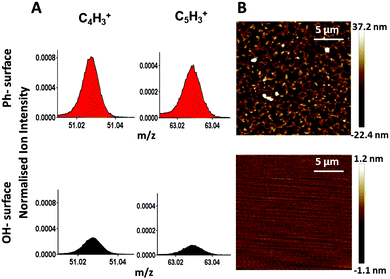
Piranha cleaned glass was used as a polar surface (WCA = 24 ± 2°) and silanisation of that glass surface with trimethoxyphenylsilane introduced phenyl groups providing a hydrophobic (WCA = 82 ± 2°) surface. ToF-SIMS analysis confirmed the presence of phenyl-groups. The linear ion fragments C4H3+ and C5H3+ have previously been reported as characteristic ToF-SIMS ions from phenyl-surfaces15 and their intensity was significantly increased on the surface after silanisation (Fig. 2A), confirming chemical modification of the surface. The topography of the surfaces was measured by AFM (Fig. 2B). The OH-surface was smooth and featureless, while the Ph-surface displayed a structured topography.
Gel films were formed on the two surfaces using the C14-cytidine (Fig. 3A) using an anti-solvent approach i.e., dissolving the gelators in ethanol and then adding the ethanol solution to water. The gelator is soluble in ethanol at the required concentration and temperature but insoluble in water. The gelator formed a fibrillar network (Fig. 3B). AFM data (see Fig. S1 in ESI†) showed that on both surfaces the C14-cytidine formed a micrometer thick gel film (1.0 ± 0.2 μm).
In order to evaluate if the gel films are homogeneous over the whole sample, AFM images were taken at extreme points across the sample and the fibre diameters were determined (Fig. S3 in ESI†). The gels' fibre diameters were found to be comparable at various locations on both surfaces, confirming that gelation occurred uniformly over the whole surface.
To establish if surface properties affect the physical and mechanical properties of the self-assembled cytidine gelator, AFM was used to measure the diameter of the self-assembled fibres as well as the Young's modulus of the gel film. When comparing the fibre diameter of both gelators on the two surfaces, significantly larger values were obtained on the Ph-surface (61.7 ± 4.4 nm) than on the OH-surface (47 ± 9 nm) (Fig. 3C). This indicates a different interaction between the gelator molecules and the surface that ultimately leads to differences in the self-assembly pathway and the molecular architecture of the gels. To assess if this effect is restricted to self-assembly processes in the proximity of the modified surface or if it extends further into the bulk of the gels we measured fibre diameters from gels prepared in vials whose surfaces were also modified to display OH and phenyl groups (Fig. S4 in ESI†). No significant differences in the fibre diameters were found, indicating that the range of the surface effect may be limited.
To investigate if the different fibre architectures are accompanied by different mechanical properties in the gel films, the relative mechanical properties (Young's modulus) of gels obtained on the two surfaces were measured via nanoindentation with AFM. Nanoindentation measurements can be used for films as thin as 1 μm16 and are therefore suitable for the present gel films (thickness: 1.0 ± 0.2 μm).
The data was plotted as histograms and fitted to a gamma distribution (p-value < 0.01). The distributions of the stiffness measurements and the skewness of the curve fits (α values) are presented in Fig. 4. The Young's moduli measured on both samples are distributed over the same range (10–600 MPa). The relatively high Young's moduli (values in the kPa range are typical for solvated gels measured in bulk17) can be explained by the fact that these gels have been dried and their mechanical properties measured as thin films on solid substrates rather than bulk conditions. The datasets for each surface were compared through the parameter α which is indicative for the skewness of the distribution. An unpaired t-test (p-value < 0.10) showed that the skewness was significantly different (t = 2.696, df = 4) on each surface. These results quantitatively demonstrate a distinct difference in the distribution of stiffness values of gel films obtained on different surfaces, indicating that gel films on Ph-surfaces (more hydrophobic and rougher) are stiffer than those on OH-surfaces (more hydrophilic, smoother).
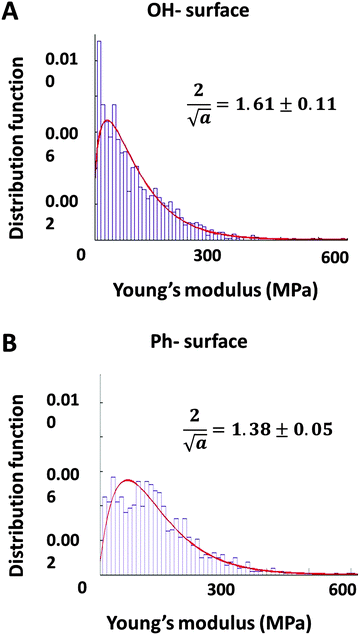 | ||
| Fig. 4 Histograms of the Young's modulus (determined by AFM) fitted with distribution functions for the C14-cytidine gel films on the OH-surface (A) and the Ph-surface (B). | ||
The effect of different surfaces on supramolecular gel film properties was investigated and quantified for the first time. We show that the gelator formed gels with different fibre diameter and different gel stiffness. This demonstrates a direct relationship between surface and gel properties, highlighting the importance of surface properties in self-assembly and providing new means for control over gel functionality.
This work was support by the EPSRC funded CDT in Targeted Therapeutics grant EP/L01646X and an RSC research bursary.
References
- L. Adler-Abramovich and E. Gazit, Chem. Soc. Rev., 2014, 43, 6881–6893 RSC.
- E. Busseron, Y. Ruff, E. Moulin and N. Giuseppone, Nanoscale, 2013, 5, 7098–7140 RSC.
- K. J. Skilling, F. Citossi, T. D. Bradshaw, M. Ashford, B. Kellam and M. Marlow, Soft Matter, 2014, 10, 237–256 RSC.
- N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- H. Wang, Z. Yang and D. J. Adams, Mater. Today, 2012, 15, 500–507 CrossRef CAS.
- R. J. Williams, R. J. Mart and R. V. Ulijn, Pept. Sci., 2010, 94, 107–117 CrossRef CAS PubMed.
- R. Huang, R. Su, W. Qi, J. Zhao and Z. He, Nanotechnology, 2011, 22, 245609 CrossRef PubMed.
- J. J. Gooding, F. Mearns, W. Yang and J. Liu, Electroanalysis, 2003, 15, 81–96 CrossRef CAS.
- M. O. Blunt, J. C. Russell, M. del Carmen Gimenez-Lopez, N. Taleb, X. Lin, M. Schröder, N. R. Champness and P. H. Beton, Nat. Chem., 2011, 3, 74–78 CrossRef CAS PubMed.
- R. J. Williams, A. M. Smith, R. Collins, N. Hodson, A. K. Das and R. V. Ulijn, Nat. Nanotechnol., 2009, 4, 19–24 CrossRef CAS PubMed.
- E. K. Johnson, L. Chen, P. S. Kubiak, S. F. McDonald, D. J. Adams and P. J. Cameron, Chem. Commun., 2013, 49, 8698–8700 RSC.
- S. S. Lee, S. B. Tang, D. M. Smilgies, A. R. Woll, M. A. Loth, J. M. Mativetsky, J. E. Anthony and Y. L. Loo, Adv. Mater., 2012, 24, 2692–2698 CrossRef CAS PubMed.
- R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519–7530 CrossRef CAS PubMed.
- K. Skilling, A. Ndungu, B. Kellam, M. Ashford, T. Bradshaw and M. Marlow, J. Mater. Chem. B, 2014, 2, 8412–8417 RSC.
- X. Dong, A. Gusev and D. M. Hercules, J. Am. Soc. Mass Spectrom., 1998, 9, 292–298 CrossRef CAS PubMed.
- J. Domke and M. Radmacher, Langmuir, 1998, 14, 3320–3325 CrossRef CAS.
- M. Oyen, Int. Mater. Rev., 2014, 59, 44–59 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, additional AFM and ToF-SIMS data and statistical analysis data. See DOI: 10.1039/c6cc00292g |
| This journal is © The Royal Society of Chemistry 2016 |