Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Crystallographic insights into (CH3NH3)3(Bi2I9): a new lead-free hybrid organic–inorganic material as a potential absorber for photovoltaics†
Kai
Eckhardt
,
Volodymyr
Bon
,
Jürgen
Getzschmann
,
Julia
Grothe
,
Florian M.
Wisser
and
Stefan
Kaskel
*
Department of Inorganic Chemistry, Technische Universität Dresden Bergstrasse 66, D-01069 Dresden, Germany. E-mail: stefan.kaskel@chemie.tu-dresden.de
First published on 19th January 2016
Abstract
The crystal structure of a new bismuth-based light-absorbing material for the application in solar cells was determined by single crystal X-ray diffraction for the first time. (CH3NH3)3(Bi2I9) (MBI) is a promising alternative to recently rapidly progressing hybrid organic–inorganic perovskites due to the higher tolerance against water and low toxicity. Single crystal X-ray diffraction provides detailed structural information as an essential prerequisite to gain a fundamental understanding of structure property relationships, while powder diffraction studies demonstrate a high degree of crystallinity in thin films.
In recent years a new class of light-absorbing materials, consisting of hybrid organic–inorganic perovskites (HOIPs), has gained tremendous attention. An editorial article published in nature materials in 2014 describes a “perovskite fever” among scientists, raising the hope that these materials might revolutionize the photovoltaic sector.1 This substance class has a three-dimensional inorganic backbone and organic cations in cuboctahedral cavities.2–4 The basic structure of the materials mainly used in the literature is CH3NH3MX3 (M = Sn, Pb and X = Cl, Br, I).2,5 These hybrid perovskites show a direct and tunable bandgap. They feature very good charge carrier mobility, high absorption coefficients and tailored electronic characteristics.6,7 The cells currently discussed in the literature show energy conversion efficiencies up to 20% proving their huge potential as an alternative to industrially produced solar cells.8 However, decisive drawbacks impede further industrial development: (1) current perovskite solar cells are based on lead and thus will fall under the category of hazardous substances with restricted use in the European Union. (2) Alternatives based on tin show a poor stability under atmospheric conditions.
Bismuth based materials could be a promising and environmentally friendly alternative. In our search for new bismuth-based materials we discovered a new methyl ammonium iodobismuthate ((CH3NH3)3Bi2I9, in the following abbreviated as MBI) with an open circuit voltage of 800 mV in a PV cell.9
In parallel Park et al. presented a similar material as an absorber with an initial efficiency of 0.12%.10
A recent workshop in February 2015 revealed that the technological advances measured by power conversion efficiencies have proceeded much more quickly than the basic physics and chemistry necessary to understand the materials.11 In this context in our current study we focus on detailed insights into the crystal structure of a new iodobismuthate and correct space group assignment. It should be mentioned that single crystals of MBI were obtained by Jakubas et al. for the first time but Weissenberg photographs only allowed to suggest the space group P63/mmc.12–14 However, Park et al. assigned their diffraction data incorrectly to a PDF number from the ICSD data base corresponding to a guanidinium salt crystallizing in Cmcm with completely different cell parameters.10
Since the correct crystal structure of MBI has not been reported yet, we synthesized crystals suitable for single crystal X-ray diffraction studies. Starting solutions of methyl ammonium iodide and bismuth(III)iodide in dimethyl-formamide (DMF) in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were used in the synthesis. The red single crystals suitable for single crystal X-ray diffraction were grown using a layer crystallization technique by separating the two starting solutions of methyl ammonium iodide and bismuth(III)iodide with dichloromethane. The single crystal X-ray diffraction study confirmed initial observations of Jakubas et al. and the crystal structure was successfully solved in the P63/mmc space group. The structure contains one independent Bi1 atom, located on the 3-fold axis (4f Wyckoff position) showing slightly distorted octahedral ligand coordination geometry (Fig. 1a and Table S1, ESI†). Two Bi atoms in the anion are coordinated by three symmetrically equivalent bridging I1 atoms (12j Wyckoff position) and terminal I2 atoms (12k Wyckoff position), both located on different mirror planes. The complex anion consists of two face-sharing octahedra, which are separated by methylammonium ions, which is a quite different situation compared with the corner sharing octahedra in the perovskite structure observed for HOIPs such as methyl ammonium lead iodide.15 A minor distortion in the BiI6 octahedra most probably originates from the repulsion of the Bi3+ ions in the Bi2I93− cluster with a Bi⋯Bi distance of 4.099(1) Å. This repulsion causes a slight deviation from octahedral angles, a contraction for the bridging I1–Bi1–I1′ (84°) and a widened I2–Bi1–I2′ angle (93°) (Table S2, ESI†). Slightly longer Bi–I1–Bi bridges (3.229(2) Å) are observed as compared to terminal Bi–I2 bonds (2.953(2) Å) as expected. In the crystal structure the anions are aligned along the c axis (Fig. 1b). The interanionic I⋯I distances range from 4.263(2) to 4.318(2) Å and are thus comparable to interlayer I⋯I contacts in elemental I2 (4.27 Å) providing a hint for potential charge transport paths. The local structure of the Bi2I93− as well as location in the unit cell is comparable with the crystal structure of tetramethylammonium bismuth iodide [(CH3)4N]3Bi2I9, crystallizing in the space group P31c.16 Because of the high symmetry of the unit cell and large difference between the scattering factors of Bi, I atoms and C, N atoms, the localization of the C and N atoms in the methylammonium cation is blurred. Both crystallographically independent cations are located on the 3-fold axis showing different type of disorder. The methyl ammonium cation (MA1) contains symmetrically equivalent C1 and N1 atoms located on the 3-fold axis close to the inversion center. In MA1 the position of the carbon and nitrogen atoms are indistinguishable causing substitutional disorder. MA2 involves two symmetrically distinguishable atoms, one of which (presumably nitrogen, N2) is located on the 3-fold axis. Another atom (presumably carbon, C2) is disordered occupying three symmetrically equivalent positions in vicinity to the 3-fold axis.
1 were used in the synthesis. The red single crystals suitable for single crystal X-ray diffraction were grown using a layer crystallization technique by separating the two starting solutions of methyl ammonium iodide and bismuth(III)iodide with dichloromethane. The single crystal X-ray diffraction study confirmed initial observations of Jakubas et al. and the crystal structure was successfully solved in the P63/mmc space group. The structure contains one independent Bi1 atom, located on the 3-fold axis (4f Wyckoff position) showing slightly distorted octahedral ligand coordination geometry (Fig. 1a and Table S1, ESI†). Two Bi atoms in the anion are coordinated by three symmetrically equivalent bridging I1 atoms (12j Wyckoff position) and terminal I2 atoms (12k Wyckoff position), both located on different mirror planes. The complex anion consists of two face-sharing octahedra, which are separated by methylammonium ions, which is a quite different situation compared with the corner sharing octahedra in the perovskite structure observed for HOIPs such as methyl ammonium lead iodide.15 A minor distortion in the BiI6 octahedra most probably originates from the repulsion of the Bi3+ ions in the Bi2I93− cluster with a Bi⋯Bi distance of 4.099(1) Å. This repulsion causes a slight deviation from octahedral angles, a contraction for the bridging I1–Bi1–I1′ (84°) and a widened I2–Bi1–I2′ angle (93°) (Table S2, ESI†). Slightly longer Bi–I1–Bi bridges (3.229(2) Å) are observed as compared to terminal Bi–I2 bonds (2.953(2) Å) as expected. In the crystal structure the anions are aligned along the c axis (Fig. 1b). The interanionic I⋯I distances range from 4.263(2) to 4.318(2) Å and are thus comparable to interlayer I⋯I contacts in elemental I2 (4.27 Å) providing a hint for potential charge transport paths. The local structure of the Bi2I93− as well as location in the unit cell is comparable with the crystal structure of tetramethylammonium bismuth iodide [(CH3)4N]3Bi2I9, crystallizing in the space group P31c.16 Because of the high symmetry of the unit cell and large difference between the scattering factors of Bi, I atoms and C, N atoms, the localization of the C and N atoms in the methylammonium cation is blurred. Both crystallographically independent cations are located on the 3-fold axis showing different type of disorder. The methyl ammonium cation (MA1) contains symmetrically equivalent C1 and N1 atoms located on the 3-fold axis close to the inversion center. In MA1 the position of the carbon and nitrogen atoms are indistinguishable causing substitutional disorder. MA2 involves two symmetrically distinguishable atoms, one of which (presumably nitrogen, N2) is located on the 3-fold axis. Another atom (presumably carbon, C2) is disordered occupying three symmetrically equivalent positions in vicinity to the 3-fold axis.
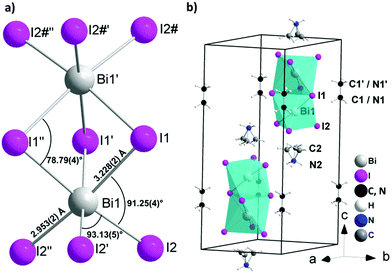 | ||
| Fig. 1 Crystal structure of (CH3NH3)3Bi2I9 (MBI): (a) local structure of the Bi2I93− anion; (b) cation and anion positions in the unit cell. | ||
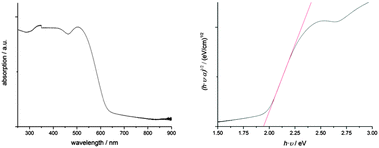
MBI has excellent absorbing properties (Fig. 2). Absorption spectra measured between 250 and 900 nm reveal an optical bandgap of the material at 1.94 eV as it was derived from a Tauc plot. This value is slightly higher than observed for BiI3 with an indirect and direct bandgap at 1.67 and 1.96 eV, respectively.17 An absorption coefficient of ∼1.1 × 105 cm−1 was estimated at a wavelength of 500 nm using an integrating sphere on MBI films with an average film thickness of 250 nm as determined from AFM measurements. Considering the roughness of MBI films, this value is in good agreement with the value given by Park et al. and is of the same order of magnitude as determined for MAPbI.3,10
XRD patterns of thin films, prepared via spin coating techniques using the solvents THF or DMF were recorded in Bragg–Brentano geometry and show good agreement when compared with the pattern calculated from single crystal data (Fig. 3) demonstrating a high degree of phase purity in the new material.
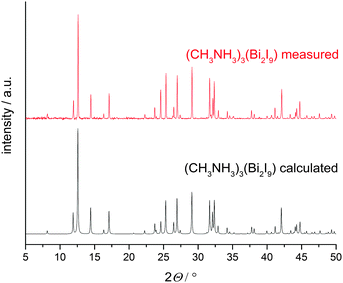 | ||
| Fig. 3 XRD patterns of (CH3NH3)3Bi2I9 calculated from single crystal data (black) compared to a measured thin film (red). | ||
Minor differences in the intensity of the peaks may be caused by preferred orientation effects of the hexagonal material in such thin films. Rietveld refinements of powder patterns give results comparable to the single crystal data.
Summarizing, we have elucidated the correct crystal structure of (CH3NH3)3Bi2I9 for the first time using single crystal measurements of this highly absorbing iodobismuthate. Even though the composition and its applicability for perovskite like hybrid solar cells would suggest similarities to CH3NH3PbI3, MBI does not contain corner sharing octahedra normally observed in perovskite materials but isolated Bi2I93− anions.‡
With the synthesis procedure described in this study we were able to show, that it is possible to obtain phase pure and highly crystalline (CH3NH3)3Bi2I9 thin films in contrast to previous work.
Integration of our MBI films in solar cell architectures following the interfaces developed for MAPbI devices, we were able to achieve a sizable open circuit voltage of ∼800 mV.18 However cell efficiencies were low, most probably due to the rough morphology of the MBI layer and the energetic mismatch within the stack structure, since it is optimized for MAPbI. Based on the structural observations reported in this work, it will be crucial to attain deeper insights into the mechanism of the charge carrier transport within MBI and optimize interfaces. Since the optical absorption coefficient of MBI and the open circuit voltage are high, MBI is worth further investigations raising the hope to offer a good alternative for lead containing absorbers. However, further work is necessary to improve processing and contacting in order to explore its full potential.
We hope that our data provide a precise basis for future studies targeting deeper insights and understanding into band structure and transport properties of this new family of Bi-based HOIP materials.
Notes and references
- L. Martiradonna, Nat. Mater., 2014, 13, 837 CrossRef PubMed.
- G. Hodes, Science, 2013, 342, 317–319 CrossRef CAS PubMed.
- M. a. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef CAS.
- S. Kazim, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Angew. Chem., 2014, 126, 2854–2867 CrossRef.
- D. B. Mitzi, Synthesis, Structure, and Properties of Organic-Inorganic Perovskites and Related Materials, John Wiley & Sons, 1994 Search PubMed.
- T. M. Koh, K. Fu, Y. Fang, S. Chen, T. C. Sum, N. Mathews, S. G. Mhaisalkar, P. P. Boix and T. Baikie, J. Phys. Chem. C, 2014, 118, 16458–16462 CAS.
- A. Kojima, M. Ikegami, K. Teshima and T. Miyasaka, Chem. Lett., 2012, 41, 397–399 CrossRef CAS.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. Il Seok, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- K. Eckhardt, Bleifreie Licht Absorbierende Verbindung, Verfahren Zu Deren Herstellung Und Deren Verwendung in Optoelektonischen Bauelementen, n.d., 10 2015 213 100.9.
- B.-W. Park, B. Philippe, X. Zhang, H. Rensmo, G. Boschloo and E. M. J. Johansson, Adv. Mater., 2015, 9, 6806–6813 CrossRef PubMed.
- J. Berry, T. Buonassisi, D. A. Egger, G. Hodes, L. Kronik, Y.-L. Loo, I. Lubomirsky, S. R. Marder, Y. Mastai and J. S. Miller, et al. , Adv. Mater., 2015, 27, 5102–5112 CrossRef CAS PubMed.
- R. Jakubas, J. Zaleski and L. Sobczyk, Ferroelectrics, 1990, 108, 109–114 CrossRef CAS.
- R. Jakubas and L. Sobczyk, Phase Transitions, 1990, 20, 163–193 CrossRef CAS.
- T. Kawai, A. Ishii, T. Kitamura, S. Shimanuki, M. Iwata and Y. Ishibashi, J. Phys. Soc. Jpn., 1996, 65, 1464–1468 CrossRef CAS.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019–9038 CrossRef CAS PubMed.
- C. Feldmann, Z. Kristallogr., 2001, 216, 465–466 CAS.
- N. J. Podraza, W. Qiu, B. B. Hinojosa, H. Xu, M. A. Motyka, S. R. Phillpot, J. E. Baciak, S. Trolier-McKinstry and J. C. Nino, J. Appl. Phys., 2013, 114, 033110 CrossRef.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details, single crystal X-ray structure analysis details. CCDC 1433118. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc10455f |
| ‡ Crystal data for (CH3NH3)3Bi2I9: C3H18Bi2I9N3, Mr = 1656.26, hexagonal P63/mmc (No. 194), a = 8.5843(12) Å, c = 21.690(4) Å, V = 1384.2(5) Å3, Z = 2, Dc = 3.974 g cm−3, 764 independent reflections observed, R1 = 0.0453 (I > 2σ(I)), wR2 = 0.1250 (all data), and GOF = 0.865; CCDC 1433118 (CH3NH3)3Bi2I9. |
| This journal is © The Royal Society of Chemistry 2016 |