Folic acid conjugated magnetic iron oxide nanoparticles for nondestructive separation and detection of ovarian cancer cells from whole blood
Wenting
Liu†
ab,
Liju
Nie†
ab,
Fulai
Li
b,
Zoraida P.
Aguilar
c,
Hong
Xu
c,
Yonghua
Xiong
b,
Fen
Fu
*a and
Hengyi
Xu
*b
aThe Second Affiliated Hospital to Nanchang University, Nanchang 330006, China. E-mail: fu_fen@163.com; Tel: +86-791-8671-6961
bState Key Laboratory of Food Science and Technology, Nanchang University, Nanchang 330047, China. E-mail: kidyxu@163.com; HengyiXu@ncu.edu.cn; Fax: +86-791-8830-4400; Tel: +86-791-8830-4447-ext-9512
cOcean NanoTech, LLC., Springdale, AR72764, USA
First published on 19th October 2015
Abstract
Because of the lack of early screening strategies, ovarian cancer is the most deadly cause of gynecologic malignancies. This paper describes an effective method for the separation and detection of ovarian cancer cells from female whole blood, using folic acid (FA) conjugated magnetic iron oxide nanoparticles (IO–FA nanoparticles). The IO nanoparticles were synthesized by thermal decomposition and then covalently conjugated with FA. The IO–FA nanoparticles were stably attached to the surface of ovarian cancer cells by coupling to the over-expressed folate receptor (FR), thereby making the cells magnetic. These “magnetic cells” were separated from the complex blood matrix without destruction under a magnetic field. The separation efficiency was as high as 61.3% when the abundance of spiked ovarian cancer SKOV3 cells was as low as 5 × 10−5%. We also successfully detected five (5) out of ten (10) metastatic ovarian cancer patients’ whole blood. This study suggested the feasibility of early detecting of metastatic ovarian cancer cells, which may potentially improve the ovarian cancers patients’ overall survival rate for clinical applications.
1. Introduction
Despite advances in chemotherapy and surgery, ovarian cancer (OC) remains a leading cause of death from gynecologic malignancies. A total of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 new OC cases with 14
240 new OC cases with 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 deaths occurred in the United States in 2013.1 When diagnosed in stage I, the 5-year survival rate of women undergoing therapy can reach up to 90%.2 Unfortunately, over three-quarters of the diagnoses are confirmed with regional or distant metastases due to the absence of specific early warning signs. Early detection of OC is still a huge challenge for clinical workers. Because the ovary is located deep in the pelvis and cannot be felt easily from the outside, there are only a few specific symptoms for OC which are not obvious. In addition, there are many limitations of existing clinical detection methods that tend to lead to misdiagnosis. For example, routine gynecological examination is insensitive when it comes to early cancer detection.3 Common clinical imaging modalities, including ultrasound imaging, magnetic resonance imaging (MRI) as well as the electronic computer X-ray tomography imaging (CT) are also insensitive in detecting tumors and metastases that are smaller than 0.5 cm and are incapable of distinguishing between benign and malignant tumors.4 Serum markers such as cancer antigen 125 (CA125) are of limited effectiveness for early detection. For example, CA125 elevated in patients with endometriosis, other diseases or benign conditions, and the baseline expressions are widely different.5 Thus, there is a significant need for an effective early screening strategy to reduce OC recurrence probability and mortality.
030 deaths occurred in the United States in 2013.1 When diagnosed in stage I, the 5-year survival rate of women undergoing therapy can reach up to 90%.2 Unfortunately, over three-quarters of the diagnoses are confirmed with regional or distant metastases due to the absence of specific early warning signs. Early detection of OC is still a huge challenge for clinical workers. Because the ovary is located deep in the pelvis and cannot be felt easily from the outside, there are only a few specific symptoms for OC which are not obvious. In addition, there are many limitations of existing clinical detection methods that tend to lead to misdiagnosis. For example, routine gynecological examination is insensitive when it comes to early cancer detection.3 Common clinical imaging modalities, including ultrasound imaging, magnetic resonance imaging (MRI) as well as the electronic computer X-ray tomography imaging (CT) are also insensitive in detecting tumors and metastases that are smaller than 0.5 cm and are incapable of distinguishing between benign and malignant tumors.4 Serum markers such as cancer antigen 125 (CA125) are of limited effectiveness for early detection. For example, CA125 elevated in patients with endometriosis, other diseases or benign conditions, and the baseline expressions are widely different.5 Thus, there is a significant need for an effective early screening strategy to reduce OC recurrence probability and mortality.
Previous research studies showed that circulating tumor cells (CTCs) in the peripheral blood of patients originated from early or metastatic tumors, which could present a window for the early detection of asymptomatic OC.6–8 The detection of CTCs could provide a sensitive and minimally invasive way for early diagnosis and prognosis evaluation, especially when the primary tumor is difficult to detect with currently available methods. However, the concentration of CTCs is extremely low, ranging from 1 per billion to 1 per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nucleated blood cells. Hence, various techniques have been explored for the capture, enrichment, and detection of CTCs.9–13 The current common clinical CTC enrichment procedures are filtration, immunomagnetic procedures with antibodies against either the common leukocyte antigen CD45 (negative selection) or the tumor-associated antigens (positive selection) and density gradient centrifugation.7 However, most of these methods are limited by their slow separation rates or the requirement for complicated pre-treatment. These limitations may damage the rare CTCs and make them more difficult to detect. So far, approaches to detect CTCs can be classified into PCR-based and cytometric methods. PCR-based methods have a high false-positive rate because the high sensitivity not only reveals the specific genes of cancer cells but also the expression of illegitimate transcripts in peripheral-blood leukocytes, the presence of mRNA in normal cells circulating at a low frequency, or contamination in blood samples during venipuncture. It is also error-prone for low-quality samples (one tumor cell usually contains only a single copy of the target gene).14 Common cytometric methods such as immunocytochemistry and fluorescence-activated flow cytometry have been widely used on samples, but it may lead to a high rate of false-negatives because of the need for specific antibodies and the elimination of interferences. As a result, enrichment and detection of rare CTCs in patients’ peripheral whole blood remains a technical challenge.6
000 nucleated blood cells. Hence, various techniques have been explored for the capture, enrichment, and detection of CTCs.9–13 The current common clinical CTC enrichment procedures are filtration, immunomagnetic procedures with antibodies against either the common leukocyte antigen CD45 (negative selection) or the tumor-associated antigens (positive selection) and density gradient centrifugation.7 However, most of these methods are limited by their slow separation rates or the requirement for complicated pre-treatment. These limitations may damage the rare CTCs and make them more difficult to detect. So far, approaches to detect CTCs can be classified into PCR-based and cytometric methods. PCR-based methods have a high false-positive rate because the high sensitivity not only reveals the specific genes of cancer cells but also the expression of illegitimate transcripts in peripheral-blood leukocytes, the presence of mRNA in normal cells circulating at a low frequency, or contamination in blood samples during venipuncture. It is also error-prone for low-quality samples (one tumor cell usually contains only a single copy of the target gene).14 Common cytometric methods such as immunocytochemistry and fluorescence-activated flow cytometry have been widely used on samples, but it may lead to a high rate of false-negatives because of the need for specific antibodies and the elimination of interferences. As a result, enrichment and detection of rare CTCs in patients’ peripheral whole blood remains a technical challenge.6
Magnetic iron oxide (IO) nanoparticles have promising potential in the isolation and detection of trace amounts of analytes because of their high surface-to-volume ratio, excellent enrichment capability, and biocompatibility15 which potentially allows highly efficient non-destructive analyte isolation. In this research, we demonstrated an effective method to separate and detect circulating OC cells from patients’ whole blood using IO–folic acid (IO–FA) nanoparticles under a low magnetic field gradient. The small size IO nanoparticle (25 nm) was able to make contact with the surface of the cells more efficiently and prevent the cells from tearing into pieces at the time of separation. The IO nanoparticles were synthesized by a pyrolysis-based method and coated with polymers to obtain the water soluble and biocompatible form. For cell capture, the IO nanoparticles were modified with FA which was used as a targeting ligand for OC cells that are rich in FR. When compared with antibodies (e.g. EpCAM), FA has many advantages: (1) FA (vitamin B9) is essential for the eukaryotic cell in the nuclear glucoside synthesis as a one-carbon donor. Moreover, it is a co-enzyme for the DNA synthesis and is utilized by various enzymatic systems.16,17 Therefore, FA is less immunogenic and safe enough for use in the human body. (2) The expression of folate receptor (FR), especially FR α, is much higher in many epithelial tumors relative to normal tissues. It was reported that 90% of OC solid tumors over expressed FR.18 (3) FA has a high affinity ligand for the FR (Kd = 0.1 nM)19, so that IO–FA nanoparticles could bind to FR found on cell surfaces efficiently and stably. (4) It is cost-effective and easy to store. In addition, the specificity of the IO–FA nanoparticles for targeting OC cells was successfully monitored by Prussian blue staining. Furthermore, IO–FA nanoparticles showed outstanding capture efficiency for OC cells in female fresh whole blood without any pre-treatment process. Finally, the identification and characterization of OC cells involved an extremely sensitive and specific analysis. Human epididymis protein 4 (HE4) has been illustrated to be over-expressed in OC cells and exhibits an advantage over the CA125 assay because it is less frequently positive in patients with other diseases.20,21 Thus, the isolated OC cells were identified with a simple QD-based immunofluorescence staining using the OC specific marker HE4. The scheme for OC cell separation and detection from the whole blood is shown in Fig. 1.
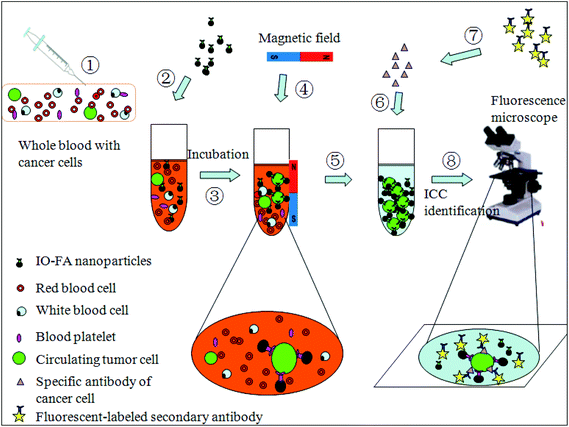 | ||
| Fig. 1 Testing scheme of OC cell separation and detection from whole blood using IO–FA nanoparticles. | ||
2. Materials and methods
2.1 Materials
SKOV3, OC cell lines with FR-positive cell surface were gifted from the Medical Research Center of the First Affiliated Hospital to Nanchang University. Lung cancer A549 cell lines with FR-negative cell surface were gifted from Jiangxi Academy of Medical Science.22 OC patients and normal female whole blood containing ethylenediaminetetraacetic acid (EDTA) as anticoagulant were obtained from the First Affiliated Hospital to Nanchang University, the Second Affiliated Hospital to Nanchang University, Jiangxi Maternal and Child Health Hospital, and Jiangxi Provincial Cancer Hospital. Albumin bovine V (BSA) was purchased from BIOSHARP (Hefei, China). Folate-free RPMI 1640 culture media was purchased from GIBCO (Grand Island, NY). The fetal bovine serum (FBS) and trypsin were from Trans Gen Biotech (Beijing, China). Goat polyclonal anti-HE4 (C-12) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Fluorescein isothiocyanate (FITC) conjugated AffiniPure Rabbit Anti-goat IgG was from EarthOx LLC (Millbrae, CA). All other chemicals of analytical grade were provided by SangonBiotech (Shanghai, China).2.2 Synthesis of IO nanoparticles
The IO nanoparticles were synthesized by thermal decomposition following the experimental procedures reported elsewhere with iron oxide powder as the iron precursor, oleic acid as the ligand, and octadecene as the solvent.23 These hydrophobic IO nanoparticles were coated with amphiphilic polymers as reported previously.24 In brief, a polymer/IO ratio of 5–10 was used, after vacuum drying, the encapsulated IO nanoparticles were suspended in a polar solvent (aqueous buffer) and then purified.2.3 Conjugation of IO nanoparticles with FA
Before conjugation with the IO nanoparticles, FA was first conjugated to diamine PEG (polyethylene glycol, PEG) to increase the flexibility of FA for optimal recognition between FA and its receptor, FR, and offer the amine group (–NH2) to covalently attach to the carboxyl group (–COOH) on the IO nanoparticles. In brief, 100 mg of FA in 10 mL dimethylsulfoxide (DMSO) was activated with 50 mg of dicyclohexylcarbodiimide (DCC) in the presence of 1 mL of triethylamine and 50 mg of N-hydroxysuccinimide (NHS) at room temperature in the dark for 4 hours, in which 0.5 g of diamine PEG was added to achieve the FA/DCC/NHS/PEG ratio of 1/1.1/1.1/1. This reaction continued overnight at room temperature in the dark. The by-product of this reaction, dicyclohexylurea was removed by centrifugation at 4000 rpm for 5 min. For purification, 10 times volume of cold acetone (−20 °C) was added to the reaction mixture to precipitate the FA-PEG-NH2. The FA-PEG-NH2 was collected by centrifugation at 4000 rpm for 15 min and the pellet was washed three times with ethyl acetate. The pellet was dried in the fume hood and stored in the dark.The FA-PEG-NH2 was conjugated to the IO nanoparticles in the following manner: 1 mg of 25 nm IO nanoparticles dissolved in 0.5 mL borate buffer (200 mM, pH = 7) was activated with 0.2 mg of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimidehydrochloride (EDC) and 0.1 mg NHS, after which 7 mg of FA-PEG-NH2 dissolved in 0.5 mL borate buffer (200 mM, pH = 7) was added. The reaction was incubated at room temperature for 2 hours with continuously stirring. The resulting IO-PEG-FA (or simply, IO–FA) nanoparticles were purified using a magnetic separator with a magnetic field gradient of 1.0 T.
2.4 Characterization of IO–FA and IO nanoparticles
The shape and size of the IO nanoparticles were observed under transmission electron microscopy (TEM). The hydrodynamic diameter and the zeta potential of the IO and IO–FA in ultrapure water were measured using a Laser particle size analyzer (Malvern, England). For the IO nanoparticle gel electrophoresis, 1% (w/v) agarose gel in a 0.5 × TBE buffer system was run at a voltage of 90 V for 15 min and 20% glycerol was mixed with the samples before loading. Fourier transform infrared spectroscopy (FTIR) (Thermo Nicolet, USA) was used to confirm the covalent bond.2.5 Cell culture
SKOV3 cells and A549 cells were cultured in culture flasks that contained 15 mL folate-free RPMI-1640 medium supplemented with 10% FBS under a humidified 5% CO2 atmosphere at 37 °C. When the cells became 80% confluent, they were detached from the cell culture flask with trypsin and rinsed once with PBS. The cells were harvested by centrifugation at 1000 rpm for 1 min and the cell pellet was re-suspended in PBS before storage at 4 °C.2.6 Specificity test of IO–FA nanoparticles by Prussian blue staining
SKOV3 cells and A549 cell lines, with 80% confluence were respectively detached and harvested with centrifugation at 1000 rpm for 1 min. The cell pellet was re-suspended in 90 μL PBS after being washed once with PBS. Two aliquots of cells were separately incubated with 50 μL IO nanoparticles and IO–FA nanoparticles at room temperature for 2 hours and washed twice with PBS, respectively. The cells were recovered by centrifugation and re-suspended in 25 μL PBS. To these, 25 μL freshly prepared Prussian blue stain solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 10% potassium ferrocyanide(II) trihydrate and 20% HCl) was added. The cell–Prussian blue mixture was incubated at 37 °C for 30 min and the stained cells were washed once with PBS before observation under a microscope.
1 mixture of 10% potassium ferrocyanide(II) trihydrate and 20% HCl) was added. The cell–Prussian blue mixture was incubated at 37 °C for 30 min and the stained cells were washed once with PBS before observation under a microscope.
2.7 Separation of spiked OC cells from whole blood using IO–FA nanoparticles
The whole blood from healthy female volunteers was spiked with SKOV3 cells at about 450 cells per mL. The cultured cells were pre-stained with fluorescent dye Hoechst33342 and counted as follows: Hoechst33342 stain was added into the SKOV3 cells at a concentration of 1 μL mL−1. After incubation for 30 min, the cells were washed three times with PBS and counted using a hemocytometer.25 A 100 μg aliquot of IO–FA nanoparticles was mixed with the spiked blood (1 mL) at room temperature for 2 h, which was then placed on a magnetic separator with a magnetic strength of 1.0 T for 4 h. The blood was pipetted out carefully, and the pellet containing IO–FA nanoparticle captured cells was re-suspended in 25 μL PBS for cell counting under a fluorescence microscope. Wright's stain was used to inspect and identify the captured cells from the whole blood in a strong magnetic field following the manufacturer's protocol. The number of SKOV3 cells spiked in the whole blood was extremely small compared to the cell population in human whole blood. After application of Wright's stain, the samples were smeared on a slide and observed using the oil immersion lens of the microscope. Moreover, a recovery test has been performed using 1 mL normal human whole blood spiked with SKOV3 (19.5 ± 2.5, 67.5 ± 7.5, 143 ± 45, 243 ± 11.5, 305 ± 9.0 cells), and each recovery has been calculated.2.8 Detection of OC cells
In order to test the ability of our method in detecting OC cells in patients’ whole blood, a total of 10 metastatic OC patients’ whole blood (serous or endometrioid ovarian carcinomas) without any pre-treatment were chosen and subjected to the IO–FA protocol. After being isolated from the blood and re-suspension as above, the cells were smeared, dried, and fixed with methanol at −10 °C. The fixed cells were blocked with 5% BSA in PBS and washed after 20 min. The isolated cells were incubated with anti-HE4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 diluted in PBS) for 60 min, washed three times with PBS, and incubated with FITC-AffiniPure Rabbit Anti-goat IgG (1
50 diluted in PBS) for 60 min, washed three times with PBS, and incubated with FITC-AffiniPure Rabbit Anti-goat IgG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 diluted in PBS) for 30 min and observed under a fluorescence microscope after being mounted with 90% glycerol in PBS.
100 diluted in PBS) for 30 min and observed under a fluorescence microscope after being mounted with 90% glycerol in PBS.
2.9 Statistical analysis
All statistical analysis was performed using SPSS 17.0 software. All tests were repeated independently in triplicates.3. Results and discussion
3.1 Characterization of IO and IO–FA nanoparticles
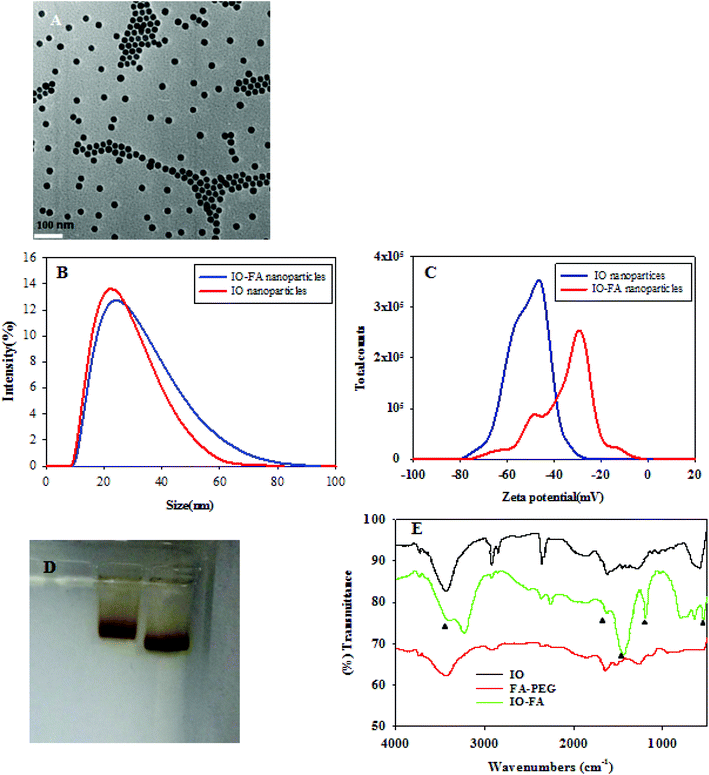
IO nanoparticles were successfully synthesized by the thermal decomposition method. Since IO nanoparticles are hydrophobic, an amphiphilic polymer was coated to convert them into the biocompatible water soluble form, which can be easily conjugated with proteins, peptides or other amine-containing molecules.26 The IO nanoparticles were characterized in terms of their size and monodispersity before use. The TEM images of the IO nanoparticles coated with the amphiphilic polymer (Fig. 2A) showed that the particles were spherical with an average inorganic core of 25 nm in diameter. The DLS images (Fig. 2B and C) showed the change in IO nanoparticle size before and after conjugation with FA. After formation of the IO–FA, the IO nanoparticles showed an increased hydrodynamic size, but the increase was not significant due to the low molecular weight of FA-PEG-NH2. The zeta potential of the IO–FA nanoparticles increased to −32.6 mV compared to the IO before conjugation which was −51.1 mV (Fig. 2C). This may be attributed to the decrease in the exposed negative carboxyl groups which reacted with the FA-PEG-NH2 during conjugation. The change in molecular mobility of the IO nanoparticles before and after conjugation with FA was also demonstrated on the gel electrophoresis (Fig. 2D). The IO–FA nanoparticles moved slower than the IO nanoparticles, indicating a higher molecular weight and a less negative zeta potential. The conjugation of FA with IO nanoparticles was further confirmed by FTIR (Fig. 2E). The FTIR absorbance vibrational peak at 541 cm−1 in both IO and IO–FA spectra corresponded to the Fe–O bond, while significant peaks at 1178 cm−1, 1623 cm−1 and 3400 cm−1 in the IO–FA spectrum may be attributed to the C–N stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch and N–H stretch from the amide linkage formed between the FA-PEG-NH2 and IO nanoparticles. Furthermore, the peaks at 1623 cm−1 and 1430 cm−1 in the FTIR of IO–FA spectrum also corresponded to the aromatic ring stretch of the pteridine ring and p-amino benzoic acid moieties of FA.27 These results revealed that the FA-PEG-NH2 was bound on the surface of the IO nanoparticles successfully.
O stretch and N–H stretch from the amide linkage formed between the FA-PEG-NH2 and IO nanoparticles. Furthermore, the peaks at 1623 cm−1 and 1430 cm−1 in the FTIR of IO–FA spectrum also corresponded to the aromatic ring stretch of the pteridine ring and p-amino benzoic acid moieties of FA.27 These results revealed that the FA-PEG-NH2 was bound on the surface of the IO nanoparticles successfully.
3.2 IO–FA nanoparticles specifically attached to the SKOV3 cells
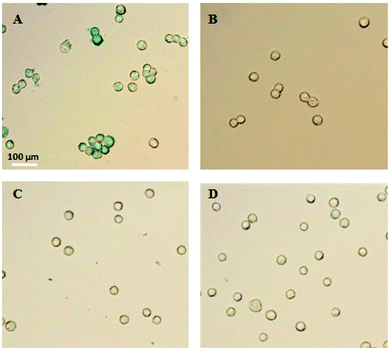
In order to demonstrate the specificity of the IO–FA nanoparticles for capturing SKOV3 cells, Prussian blue staining was used which reacts with iron. When IO–FA nanoparticles attached to the surface of SKOV3 cells successfully, the potassium ferrocyanide (K4Fe(CN)6) reacts with the iron ion from the IO nanoparticles and forms ferrous ferricyanide (Fe4[Fe(CN)6]3) which appears as a dark blue coloration. The control group composed of A549 cell lines which do not express FR and IO-nanoparticles without conjugated FA was also subjected to the same tests. As shown in Fig. 3A, SKOV3 cells incubated with IO–FA nanoparticles were specifically labeled blue while SKOV3 cells incubated with IO nanoparticles without FA, A549 cells that lacked FR incubated with IO–FA nanoparticles, or A549 cells incubated with IO nanoparticles, showed insignificant blue coloration (Fig. 3B–D, respectively). These results indicated that IO–FA nanoparticles were specifically attached to the SKOV3 cells through the FA–FR interaction with minimal to no non-specific interaction.3.3 Selective capture of spiked OC cells from whole blood using IO–FA nanoparticles
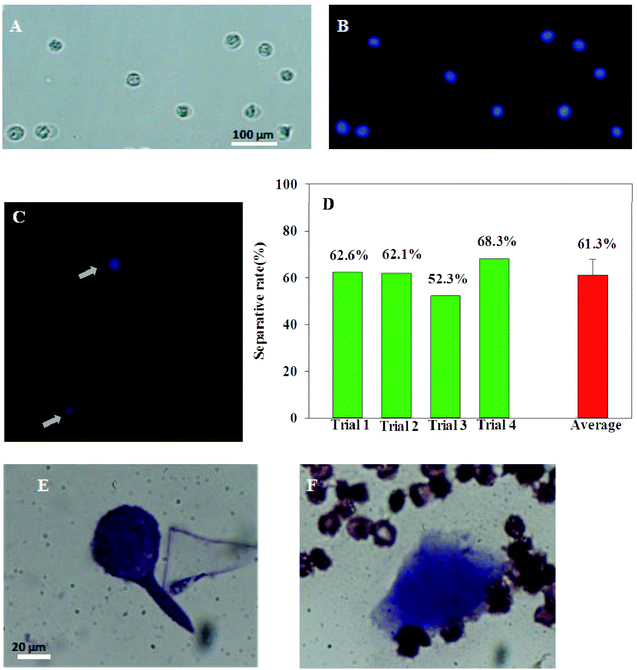
To evaluate the efficiency of these IO–FA nanoparticles in the selective capture of OC CTCs, enrichment experiments were carried out using whole blood spiked with SKOV3 cells. In order to identify the SKOV3 cells from the cells in the blood matrix, SKOV3 cells were pre-stained with the fluorescent dye Hoechst33342. As shown in Fig. 4A and B, SKOV3 cells stained well and had long-term stability and brightness. Blood, a complex medium containing a variety of cells and components including billions of red blood cells and white blood cells, has an average number of white blood cells in healthy women's whole blood at (4–10) × 109 cells per L and red blood cells at (3.5–5) × 1012 cells per L. In order to replicate the CTCs in the human blood circulation system, 450 cancer cells were mixed with 1 mL of the whole blood sample from women at a ratio of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. IO–FA nanoparticles were added to selectively capture the spiked SKOV3 cells. As shown in Fig. 4C, SKOV3 cells were successfully captured from the whole blood, and were easily identified with strong blue fluorescence under a fluorescence microscope. The efficiency of these IO–FA nanoparticles in the selective separation of the spiked SKOV3 cells ranged from 52.3% to 68.3% (N = 4), with an average of 61.3% (Fig. 4D). The results clearly indicated the promising capabilities of the IO–FA nanoparticles in enriching OC cells with high levels of FR expression from whole blood. Also, the feasibility of the method for using FA coated MNPs to enrich and detect CTCs from whole blood was confirmed with the recovery test, and the results showed 42.66% to 65.18% recovery with 19.5 ± 2.5 to 305 ± 9.0 cells per mL.
000. IO–FA nanoparticles were added to selectively capture the spiked SKOV3 cells. As shown in Fig. 4C, SKOV3 cells were successfully captured from the whole blood, and were easily identified with strong blue fluorescence under a fluorescence microscope. The efficiency of these IO–FA nanoparticles in the selective separation of the spiked SKOV3 cells ranged from 52.3% to 68.3% (N = 4), with an average of 61.3% (Fig. 4D). The results clearly indicated the promising capabilities of the IO–FA nanoparticles in enriching OC cells with high levels of FR expression from whole blood. Also, the feasibility of the method for using FA coated MNPs to enrich and detect CTCs from whole blood was confirmed with the recovery test, and the results showed 42.66% to 65.18% recovery with 19.5 ± 2.5 to 305 ± 9.0 cells per mL.
In order to identify and observe the morphological changes of these cancer cells after magnetic separation from whole blood, Wright's stain was used. Wright's stain is commonly used in clinical practice to reveal the cell structure and to distinguish different types of cells. Cultured SKOV3 cells stained with Wright stain were big in size with a large, irregular, and hyperchromatic nucleus (Fig. 4E). The morphology of SKOV3 cells was very different from the blood cells, after being isolated by IO–FA nanoparticles with the magnet, the captured cells were intact and the same as the cultured cells (Fig. 4F). These results indicated that the IO–FA nanoparticles specifically bound to the SKOV3 cells through the FA–FR interaction, which rendered the cells magnetic without visible morphological damage. Therefore, it may be feasible to effectively capture and detect cancer cells from the circulation in peripheral blood for early diagnosis of OC.
3.4 Detection of OC cells in peripheral blood of patients with metastatic OC
In order to test the ability of the IO–FA method of isolating OC cells in patients’ whole blood, a total of 10 metastatic OC patients’ whole blood (serous or endometrioid ovarian carcinomas) without any pre-treatment were used. Immunofluorescence staining was used to confirm the capture of the OC cells through the biomarker HE4 which is a widely recognized tumor marker that is over-expressed in OC cells, especially in serous and endometrioid ovarian carcinomas.28 The OC cells were fluorescent green and bigger in size than the blood cells under the fluorescence microscope as shown in Fig. 5. The results indicated successful detection of the OC cells in 5 samples from 10 metastatic OC patients’ whole blood. However, further studies must be carried out to improve the recovery, efficiency, and accuracy of the chosen detection method. | ||
| Fig. 5 Immune fluorescence images of OC cell isolated with the IO–FA from a cancer patient's whole blood under a fluorescence microscope. | ||
4. Conclusion
Early diagnosis of OC remains a problem for clinical treatment. Although magnetic nanomaterials have been widely used in the detection of tumor cells, which holds promise for the early diagnosis of OC, separating cancer cells from the whole blood directly still faces a lot of difficulties such as low separation efficiency, process complexity, and pre-treatment requirement (thinning the blood or incubating cancer cells with magnetic nanoparticles of various sizes).29–32 In this report, we demonstrated the feasibility of using 25 nm IO–FA nanoparticles for nondestructive OC cell enrichment and detection from whole blood. After being conjugated with FA, the IO nanoparticles attached to the surface of OC cells efficiently and securely, allowing separation from the complex human whole blood without destructive effects to the cell morphology with the use of a strong magnetic field. The separation efficiency was as high as 61.3% when the abundance of cancer cells was as low as 450 cells in 1 mL of blood which translates to 5 × 10−5% CTCs in whole blood, which was much higher than other published methods.30,32 Out of 10 metastatic OC patients’ whole blood, cancer cells were successfully detected in 5 samples by the new IO–FA method. The results of this study suggested the feasibility of using IO–FA nanoparticles for the isolation of CTCs for the early detection of metastatic OC.Acknowledgements
This project was supported by the National Natural Science Foundation of China (81201691 and 31271863), the Science and Technology Planning Project of Jiangxi Province (20151BBG70077), the research fund for the Doctoral Program of Higher Education of China (20123601120005), and the Training Plan for the Young Scientist (Jinggang Star) of Jiangxi Province (20142BCB23004).References
- R. Siegel, D. Naishadham and A. Jemal, Cancer statistics, 2013, CA-Cancer J. Clin., 2013, 63(1), 11–30 CrossRef PubMed.
- D. Badgwell and R. C. Bast Jr., Early detection of ovarian cancer, Dis. Markers, 2007, 23(5), 397–410 CrossRef CAS PubMed.
- P. M. Das and R. C. Bast Jr., Early detection of ovarian cancer, 2008 Search PubMed.
- R. Popovtzer, et al., Targeted gold nanoparticles enable molecular CT imaging of cancer, Nano Lett., 2008, 8(12), 4593 CrossRef CAS PubMed.
- Y.-E. Choi, J.-W. Kwak and J. W. Park, Nanotechnology for early cancer detection, Sensors, 2010, 10(1), 428–455 CrossRef CAS PubMed.
- L. Joseph, Circulating Tumor Cells and Nucleic Acids for Tumor Diagnosis, in Molecular Pathology of Neoplastic Gastrointestinal Diseases, Springer, 2013, pp. 229–247 Search PubMed.
- K. Pantel and C. Alix-Panabières, Circulating tumour cells in cancer patients: challenges and perspectives, Trends Mol. Med., 2010, 16(9), 398–406 CrossRef PubMed.
- M. Yu, et al., Circulating tumor cells: approaches to isolation and characterization, J. Cell Biol., 2011, 192(3), 373–382 CrossRef CAS PubMed.
- E. Racila, et al., Detection and characterization of carcinoma cells in the blood, Proc. Natl. Acad. Sci. U. S. A., 1998, 95(8), 4589–4594 CrossRef CAS.
- K. E. Scarberry, et al., Magnetic nanoparticle− peptide conjugates for in vitro and in vivo targeting and extraction of cancer cells, J. Am. Chem. Soc., 2008, 130(31), 10258–10262 CrossRef CAS PubMed.
- A. A. Adams, et al., Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor, J. Am. Chem. Soc., 2008, 130(27), 8633–8641 CrossRef CAS PubMed.
- M. Ntoulia, et al., Detection of Mammaglobin A-mRNA-positive circulating tumor cells in peripheral blood of patients with operable breast cancer with nested RT-PCR, Clin. Biochem., 2006, 39(9), 879–887 CrossRef CAS PubMed.
- C.-H. Ohlmann, et al., Detection of circulating tumor cells in patients with renal cell carcinoma by reverse transcriptase polymerase chain reaction for G250/MNCA-9: results of a prospective trial, in Urologic Oncology: Seminars and Original Investigations, Elsevier, 2006 Search PubMed.
- S. Mocellin, et al., Circulating tumor cells: the ‘leukemic phase' of solid cancers, Trends Mol. Med., 2006, 12(3), 130–139 CrossRef CAS PubMed.
- Y. Cohen and S. Y. Shoushan, Magnetic nanoparticles-based diagnostics and theranostics, Curr. Opin. Biotechnol., 2013, 24(4), 672–681 CrossRef CAS PubMed.
- G. Hong, et al., Folate-functionalized polymeric micelle as hepatic carcinoma-targeted, MRI-ultrasensitive delivery system of antitumor drugs, Biomed. Microdevices, 2008, 10(5), 693–700 CrossRef CAS PubMed.
- C. Chen, et al., Structural basis for molecular recognition of folic acid by folate receptors, Nature, 2013, 500(7463), 486–489 CrossRef CAS PubMed.
- P. S. Low and S. A. Kularatne, Folate-targeted therapeutic and imaging agents for cancer, Curr. Opin. Chem. Biol., 2009, 13(3), 256–262 CrossRef CAS PubMed.
- R. I. Pinhassi, et al., Arabinogalactan− Folic Acid− Drug Conjugate for Targeted Delivery and Target-Activated Release of Anticancer Drugs to Folate Receptor-Overexpressing Cells, Biomacromolecules, 2009, 11(1), 294–303 CrossRef PubMed.
- J. B. Welsh, et al., Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer, Proc. Natl. Acad. Sci. U. S. A., 2001, 98(3), 1176–1181 CrossRef CAS PubMed.
- I. Hellström, et al., The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma, Cancer Res., 2003, 63(13), 3695–3700 Search PubMed.
- Y. Zheng, et al., Preparation and characterization of folate-poly (ethylene glycol)-grafted-trimethylchitosan for intracellular transport of protein through folate receptor-mediated endocytosis, J. Biotechnol., 2010, 145(1), 47–53 CrossRef CAS PubMed.
- W. Y. William, et al., Synthesis of monodisperse iron oxide nanocrystals by thermal decomposition of iron carboxylate salts, Chem. Commun., 2004,(20), 2306–2307 Search PubMed.
- X. Gao, et al., In vivo cancer targeting and imaging with semiconductor quantum dots, Nat. Biotechnol., 2004, 22(8), 969–976 CrossRef CAS PubMed.
- C. Kaittanis, S. Santra and J. M. Perez, Role of nanoparticle valency in the nondestructive magnetic-relaxation-mediated detection and magnetic isolation of cells in complex media, J. Am. Chem. Soc., 2009, 131(35), 12780–12791 CrossRef CAS PubMed.
- T. Pellegrino, et al., Hydrophobic nanocrystals coated with an amphiphilic polymer shell: a general route to water soluble nanocrystals, Nano Lett., 2004, 4(4), 703–707 CrossRef CAS.
- C. Sun, R. Sze and M. Zhang, Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI, J. Biomed. Mater. Res., Part A, 2006, 78(3), 550–557 CrossRef PubMed.
- R. Drapkin, et al., Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas, Cancer Res., 2005, 65(6), 2162–2169 CrossRef CAS PubMed.
- Z. Chen, et al., Graphite-Coated Magnetic Nanoparticle Microarray for Few-Cells Enrichment and Detection, ACS Nano, 2012, 6(2), 1094–1101 CrossRef CAS PubMed.
- M. Hossain, et al., X-ray enabled detection and eradication of circulating tumor cells with nanoparticles, Biosens. Bioelectron., 2012, 38(1), 348–354 CrossRef CAS PubMed.
- M. Y. Sha, et al., Surface-enhanced Raman scattering tags for rapid and homogeneous detection of circulating tumor cells in the presence of human whole blood, J. Am. Chem. Soc., 2008, 130(51), 17214–17215 CrossRef CAS PubMed.
- S. S. Banerjee, et al., Transferrin-Mediated Rapid Targeting, Isolation, and Detection of Circulating Tumor Cells by Multifunctional Magneto-Dendritic Nanosystem, Adv. Healthcare Mater., 2013, 2(6), 800–805 CrossRef CAS PubMed.
Footnote |
| † Co-first authors. |
| This journal is © The Royal Society of Chemistry 2016 |