Colorimetric and ratiometric fluorescent probe for hydrogen sulfide using a coumarin–pyronine FRET dyad with a large emission shift†
Abstract
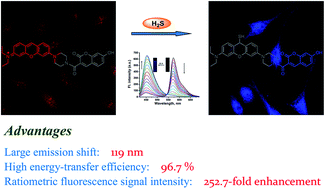
Ratiometric fluorescent probes with two well-resolved emission bands have attracted considerable attention, as they can be employed to carry out ratiometric measurements. The ratiometric measurements can alleviate to some extent the shortcomings of intensity-based probes induced by the probe concentration effects, influence of probe environment, and instrumental limitations. Herein, a colorimetric and ratiometric fluorescent probe (CP-H2S) based on a coumarin–pyronine FRET dyad was developed for the detection of H2S in vitro and in vivo. In an aqueous solution, the probe exhibits an inherent red emission of the pyronine unit excited from the coumarin unit by FRET process, whereas an encounter of CP-H2S and H2S suppresses the FRET process and elicits a new blue emission of the coumarin moiety with a concomitant decrease in the red emission. The spectral shift of these two distinct emission bands is up to 119 nm. Moreover, there was a 252.7-fold enhancement in the ratiometric fluorescence intensity signal, and the energy-transfer efficiency was found to be 96.7%. These desirable results suggest that the CP-H2S probe can significantly reduce the crosstalk signals and improve the accuracy of ratiometric measurements. Remarkable ratio of signals of the CP-H2S probe upon responding to H2S was also obtained in living cells imaging.

 Please wait while we load your content...
Please wait while we load your content...