Potentiometry for the determination of oxidant activity
Abstract
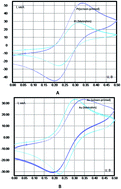
This paper aims to describe a new approach to using potentiometry for determining oxidants in liquids and ozonized and chlorinated water, in particular. The source of information is the electrode potential shift of the mediator system observed when an analyzed sample, containing oxidants, is inserted into an electrochemical cell. Criteria for the selection of the mediator system for oxidant determination are proposed. Potassium ferri/ferrocyanides serve as the mediator system in the proposed method. K3[Fe(CN)6] is chosen as the model oxidant. Special attention is focused on the role of the state of an indicator electrode surface and ways of its regeneration in generating an analytical signal. The information is essential for obtaining correct results in different areas of electrochemical analysis. As a result, a simple, fast, sensitive, and reliable method that does not require the use of reference solution is proposed, which ensures its advantages over other methods. The accuracy and reliability of the data obtained are confirmed by the analysis results received by using the standard method.

 Please wait while we load your content...
Please wait while we load your content...