Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Analysis of alkyl esters of p-hydroxybenzoic acid (parabens) in baby teethers via gas chromatography-quadrupole mass spectrometry (GC-qMS) using a stable isotope dilution assay (SIDA)
Theodoros
Potouridis
a,
Elisabeth
Berger
bc and
Wilhelm
Püttmann
*a
aInstitute for Atmospheric and Environmental Sciences, Department of Environmental Analytical Chemistry, J.W. Goethe-University Frankfurt am Main, Altenhöferallee 1, D-60438 Frankfurt am Main, Germany. E-mail: puettmann@iau.uni-frankfurt.de; Fax: +49-69-798-40240; Tel: +49-69-798-40225
bFaculty of Biology, Department Aquatic Ecotoxicology, J.W. Goethe-University Frankfurt am Main, Max-von-Laue-Str. 13, D-60438 Frankfurt am Main, Germany
cSenckenberg Research Institute and Natural History Museum Frankfurt, Department of River Ecology and Conservation, Clamecystr. 12, D-63571 Gelnhausen, Germany
First published on 14th April 2016
Abstract
Alkyl esters of p-hydroxybenzoic acid (parabens) are well-known to be used as preservatives in pharmaceuticals, cosmetics, and food products due to their antimicrobial effect. However, parabens are also known to act as endocrine disruptors. Thus, if manufactures of consumer goods use parabens particularly in products for infants and young children, this application should be considered as critical and should be thoroughly investigated, at least if an intake into the body cannot be excluded. The present work describes an analytical method for the analysis of methyl-, ethyl- and n-propylparaben (MeP, EtP and n-PrP) in plastic and gel material from baby teethers filled with cooling gel. Measurements were performed with gas chromatography-quadrupole mass spectrometry (GC-qMS) on N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) derivatized parabens. Samples were prepared applying ultrasonic assisted extraction (UAE) using methanol as the solvent. For quantification, a stable isotope dilution assay (SIDA) was used that showed good recoveries for spiked gel material, which ranged from 82 to 119%. The baby teether analyses showed that parabens were present in all considered plastic and gel samples. Furthermore, in all gel samples (2E,4E)-hexa-2,4-dienoic acid (sorbic acid) was identified as an additional preservative.
Introduction
Baby teethers are available in a wide range of models. Customers have the choice between different colours, shapes, sizes, surface structures and degrees of hardness. Moreover, some products contain a cooling gel and can be cooled in a domestic refrigerator before use so that the cooling effect may additionally contribute to the pain-relieving effect of the teether. Manufacturers or retailers often advertise further possible benefits of such baby teethers with functional characteristics like a contribution to the development of the motor skills of the infants. Instructions for use, product packaging or corresponding advertising frequently use the words “BPA free” (contains no bisphenol A) or “non-toxic” to give an overall impression of harmlessness.The products that were considered in the present study were filled with a cooling gel of unknown composition. From a microbiological point of view, the basic gel material might be critical because microbial growth is principally possible in a gel due to the high water content. For this reason, it is conceivable that preservatives were added to these gel fillings. Alkyl esters of p-hydroxybenzoic acid (parabens) are widely used as additives in pharmaceuticals, cosmetics, and food products1,2 due to their well-known antimicrobial effect.3
However, health concerns have been raised because parabens are also known to act as endocrine disruptors.4–6 In 2010 the European Scientific Committee on Consumer Safety (SCCS) recommended in an opinion letter to lower the maximum allowed concentration of propyl- and butylparaben in cosmetics.7 In 2011 the SCCS issued an opinion8 concerning the decision of the Ministry of the Environment of Denmark that banned both parabens including their isoforms and their salts from cosmetic products for children up to three years of age. The SCCS concluded that a risk from parabens in leave-on cosmetic products for application in the nappy area cannot be excluded for infants below the age of six months. Recently, the European Parliament and Council prohibited the use of iso-propyl- and iso-butylparaben and their salts, and also phenyl-, benzyl- and pentylparaben in cosmetic products.9 This decision shows clearly how concerns are taken seriously and how the authorities respond to the limited availability of data on safety evaluation. Finally, for foods for infants and young children the use of parabens for preservation is prohibited within the European Union (EU).10
In a previous research study11 about the endocrine activity of different baby teethers conducted by a cooperating working group, a specimen with estrogenic and antiandrogenic activity was identified using two in vitro bioassays, namely the Yeast Estrogen Screen (YES) and the Yeast Antiandrogen Screen (YAAS). The respective baby teether showed the presence of methyl-, ethyl- and n-propylparaben (MeP, EtP and n-PrP) in a non-target chemical analysis of the plastic material by use of gas chromatography-quadrupole mass spectrometry (GC-qMS). It was the only teether in this study that contained a cooling gel. However, the gel was not investigated and paraben concentrations were not quantified. Since quantitative data are essential for a risk assessment, the present study aimed at the quantification of the discussed paraben species both in the plastic and gel material of the named respective product. In order to figure out whether the presence of parabens is directly connected to the cooling gel, other products with gel fillings should be analyzed. Due to short-term availability problems, the additionally investigated teethers were purchased from the same manufacturer as the one that was tested positive in the previous study.
For the identification and quantification of parabens, different sample preparation methods and analytical techniques have been reported. For example, analyses of parabens in pharmaceuticals,12,13 cosmetics,14–19 and food products20–22 were performed with liquid or gas chromatography coupled to different detector types, e.g. a mass spectrometer or capillary electrophoresis system. Sample extracts were obtained by ultrasonic assisted extraction (UAE),15,22 liquid–liquid extraction (LLE),13 supercritical fluid extraction (SFE),14 solid-phase extraction (SPE),17,20 hollow-fiber liquid-phase microextraction (HF-LPME)18 and matrix solid-phase dispersion (MSPD).19 Also combined techniques of SFE with solid-phase microextraction (SPME),16 LLE with subsequent SPE20 or solid–liquid extraction (SLE) with subsequent SPE21 were used. The analytical approaches often depend on the sample type and complexity of the matrix. Therefore, appropriate sample preparation methods have to be applied in combination with instrumental analytical techniques. Particularly, if isotope-labeled compounds are used as internal standards for quantification and the capability of the chromatographic system is insufficient to separate the target compounds from their highly deuterated isotopologues, mass spectrometric detection is required to extract selective fragment ions.
Therefore, it was the aim of the present study to establish an accurate and reliable analytical method for the analysis of parabens in plastic and gel material of baby teethers by suspected-target and target screening using a simple instrumental set-up without elaborate sample preparation.
During the course of analysis, the BSTFA derivative of (2E,4E)-hexa-2,4-dienoic acid (sorbic acid) had been tentatively identified in one sample. Therefore, all samples were investigated for the presence of this additional preservative.
Furthermore, identified parabens and their detected concentrations are discussed and compared with maximum values for the preservation of cosmetic products and foods established in the EU.
Experimental
Materials and chemicals
Methyl 4-hydroxybenzoate (methylparaben, MeP; purity ≥ 99%), ethyl 4-hydroxybenzoate (ethylparaben, EtP; purity ≥ 99%), n-propyl 4-hydroxybenzoate (n-propylparaben, n-PrP; purity ≥ 99%), potassium (2E,4E)-hexa-2,4-dienoic acid (potassium sorbate; purity ≥ 99%) and sodium sulfate (purity ≥ 99%) were obtained from Aldrich (Steinheim, Germany). Methyl 4-hydroxybenzoate-2,3,5,6-[2H4] (methylparaben-d4, MeP-d4; purity 99%, isotopic enrichment 98 atom% D) was purchased from CDN Isotopes (Quebec, Canada). Methanol (purity ≥ 99.9%), dichloromethane (purity ≥ 99.5%) and hydrochloric acid 37% (HCl, extra pure) were obtained from Carl Roth (Karlsruhe, Germany). Methanol and dichloromethane were distilled in-house before use. Pyridine (purity ≥ 99.5%) was obtained from Merck (Darmstadt, Germany). The silylation reagent N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) was purchased from CS-Chromatographie (Langerwehe, Germany). Helium gas for GC was of purity 5.0 and supplied by Praxair (Düsseldorf, Germany).Samples
The gel-filled baby teethers analyzed in the present study were all from one single manufacturer and purchased from local and internet retailers in Germany between 2012 and 2014. One of these five products had the form of a soother (teether no. 5); the others were in the form of a closed ring. The baby teether that had shown endocrine activity in a previous study11 was bought again in 2013 with a different lot number (baby teether no. 2) and reanalyzed together with a sample of the original product from 2012 (baby teether no. 1). This was done to see if the findings for the baby teether purchased in 2012 were reproducible in a later produced batch.The plastic material of the chewing part of the products consisted of the copolymer type ethylene-vinyl acetate (EVA). As described in a previous study,11 this was determined via Fourier Transform Infrared Spectroscopy (FTIR) by comparison of obtained FTIR spectra with reference FTIR spectra of known plastic materials.
For all teethers, the quantitative analyses were done on the plastic and gel material.
Sample preparation
The analysis of plastic and gel material was done on 1 g, respectively. The gel material was taken from one randomly chosen spot of the baby teether. In the case of the plastic material, three pieces with a total weight of 1 g were cut from each baby teether. For UAE, all samples were weighted in 22 mL glass vials with polytetrafluoroethylene-lined screw-caps. Then, 10 mL of methanol was added, followed by sonication with an ultrasonic device (SONOREX SUPER RK 510 H, BANDELIN, Berlin, Germany) at 35 kHz of ultrasonic frequency (ultrasonic peak output: 640 W) in a water bath for one hour. At the beginning of each extraction, the water bath had a temperature in a range from 20 to 22 °C. After 1 hour extraction time the temperature had increased to 38 to 42 °C. The methanol extracts of the plastic samples were subsequently transferred to clean 22 mL glass vials. In the case of the gel material, the samples were dissolved in the used extraction solvent. For quantification some plastic and gel methanol extracts had to be further diluted.Before silylation of the target compounds with BSTFA, 2 mL aliquots of the plastic and gel methanol solution, respectively, were spiked with 2 μL of a MeP-d4 internal standard solution with a concentration of 2.46 mg mL−1 methanol, in order to carry out the quantification by a stable isotope dilution assay (SIDA). This corresponds to a concentration for MeP-d4 of 24.6 μg g−1 sample material. The principle of SIDA, its historical background, advantages over other quantification techniques, and also application examples in food analysis, have been summarized by Milo and Blank23 and Rychlik and Asam.24,25
In all prepared calibration standards and samples, parabens were converted into trimethylsilylesters with BSTFA to increase their volatility and decrease their polarity and thus to achieve a better detectability in GC analyses.26 It should be noted that the so-called short chain alkyl compounds MeP, EtP, and n-PrP could also have been analyzed underivatized. However, by silylation of the hydroxyl group, interactions of the compounds with possible active zones inside the GC system leading to peak tailing and thus integration problems can be avoided. Thereby, lower paraben concentrations are detectable and consequently lower limit of detection (LOD) and limit of quantification (LOQ) values can be achieved. Silylation of the parabens and MeP-d4 with BSTFA was done as follows. At first, 50 μL of each solution to be analyzed was transferred into a 0.8 mL conical GC vial and evaporated to dryness at 70 °C using a metal block thermostat (Thermobil TM-200-56, Liebisch, Bielefeld, Germany). Then, 10 μL BSTFA and 10 μL pyridine were added and the closed vial was placed in the metal block thermostat again for 30 min at 70 °C. Next, 200 μL dichloromethane was added and samples were analyzed with automated sample injection by GC-qMS.
GC-qMS operating conditions
The measurements were performed using a Trace GC Ultra, equipped with a split/splitless injector and an AI/AS 3000 Series II autosampler (ThermoFisher Scientific, Dreieich, Germany). The GC was coupled to a DSQ II system (quadrupole MS, ThermoFisher Scientific, Dreieich, Germany) as the mass spectrometric detector. For gas chromatographic separation, a 30 m × 0.25 mm i.d. fused silica capillary, coated with 5% diphenyl/95% dimethylpolysiloxane of 0.25 μm film thickness (ZB-5MSi, Phenomenex, Aschaffenburg, Germany) was used, with a constant carrier gas flow of 1.1 mL helium per min. GC injector port temperature was set to 240 °C. GC oven temperature was programmed from 40 °C (1 min hold time for suspected-target screening; 2 min hold time for target screening) at 25 °C min−1 up to 130 °C, at 4 °C min−1 up to 260 °C, and at 25 °C min−1 up to 300 °C (15 min hold time). Transfer line for coupling of the GC oven and qMS detector was set to 280 °C. Mass detection was done on positive ions after electron ionization at 70 eV, with an ion source temperature of 220 °C.In a first step, in order to identify the target compounds (i.e. for suspected-target screening), prepared samples were analyzed undiluted. GC sample injections of 1 μL were performed in splitless mode (split valve closed for 1 min). The mass scan range of the qMS was set in full scan mode (m/z 50–650) with a scan rate of 2 scans per s. Then, for target screening of the paraben compounds, prepared samples were analyzed undiluted or after appropriate dilution with methanol, and a calibration was established. For these measurements, injection parameters were altered to 2 μL injection volume in split mode with a split flow of 11 mL min−1. Also selected ion monitoring (SIM) mode for mass detection of qualifier and quantifier ions (bold) was applied by scanning of the following masses: m/z = 193 (197), 209 (213), 224 (228) for MeP (MeP-d4); m/z = 193, 223, 238 for EtP; m/z = 193, 237, 252 for n-PrP. The quantifier ion of MeP is the molecule ion and the fragment ions selected for quantification of EtP and n-PrP are the base peaks of the respective mass spectra (in the case of EtP the quantifier ion m/z = 193 has nearly the same intensity as the qualifier ion m/z 223). Mass scan width of all SIM masses was m/z = 1 and dwell time was set at 100 ms.
For data acquisition, instrumental operating control and analysis of the data, the Xcalibur software (version 2.0.7, ThermoFisher Scientific, Dreieich, Germany) was used. Mass spectra search of the parabens was performed by the NIST MS Search software (version 2.0, National Institute of Standards and Technology, Gaithersburg, Maryland, USA).
Identification of parabens and sorbic acid
The identification of BSTFA derivatives of MeP, EtP, n-PrP and sorbic acid in samples of suspected-target screening was based on comparison of the mass spectra and linear retention indices (LRI)27,28 with those of derivatized reference substances in a paraben calibration solution and in a prepared sorbic acid solution. LRI values of paraben derivatives on a slightly polar ZB-5MSi GC separation column were calculated according to van den Dool and Kratz.28For the analysis of sorbic acid, a potassium sorbate solution with a concentration of 10 mg 10 mL−1 ultrapure water (Astacus Analytical System, membraPure, Bodenheim, Germany) with a pH of 2 (adjusted with 1 mol L−1 HCl) was prepared and then extracted with 10 mL dichloromethane by gentle shaking for 5 min. After discarding the upper aqueous phase the organic phase containing the sorbic acid was dried using sodium sulfate. Also, a potassium sorbate solution with a concentration of 10 mg 10 mL−1 methanol was prepared and used for analysis. Subsequently, these solutions were treated as the real samples described in the section Sample preparation, however without applying UAE and without addition of MeP-d4.
Method calibration
To establish a linear calibration curve, seven concentration levels in the range of 5 to 200 μg 10 mL−1 methanol (5, 10, 25, 50, 100, 150 and 200 μg 10 mL−1) for MeP, EtP and n-PrP, respectively, were prepared and analyzed as single measurements. Each single calibration solution contained a constant concentration level of MeP-d4 (24.63 μg 10 mL−1) as the internal standard, resulting in ratios from approximately 0.2 to 8 (analyte/internal standard). Prior to adding the internal standard, the paraben calibration solutions were also treated equal to the real samples by use of UAE. The LOD and LOQ values for MeP, EtP and n-PrP were estimated by the mathematical–statistical approach according to the German standard DIN 32645 (ref. 29) based on calibration data.Method validation
Before sample preparation, quality assessment of used solvents was carried out on methanol and dichloromethane by reducing a volume of 20 mL to approximately 2 mL in a gentle stream of nitrogen at room temperature. Pre-concentrated methanol and dichloromethane were further treated as the real samples.Selected samples were used for evaluating the accuracy of the applied analytical method. The recovery and repeatability (intra-day precision) of the UAE//GC-qMS method for analysis of gel material were determined on a set of five independently spiked samples and five original samples of baby teether no. 3, respectively. Individual standard solutions consisting of approximately 250 mg (±3 mg) of MeP, EtP, and n-PrP in 5 mL methanol were prepared and 3 μL of each solution was used for spiking of 1 g material, corresponding to approximately 150 μg g−1 gel material. In order to assess the recovery performance of the method at different concentration levels, the spiked samples were diluted for quantification to cover the high, medium and low (close to LOQ values) concentration levels of the calibration curves.
The repeatability (intra-day precision) of the UAE//GC-qMS method for the analysis of plastic material was performed by measuring two different sets of five samples each prepared independently from two different baby teethers, using samples no. 1 for MeP and n-PrP and no. 3 for EtP. Recovery could not be determined for the plastic material, since there was no possibility of spiking the present material nor could any reference material with certified paraben contents be obtained.
To determine the repeatability over several days (inter-day precision), the prepared sample sets described above were additionally analyzed on three further days within one week.
Procedural blanks (i.e. sample preparation applied on methanol only) were conducted for each plastic and gel sample batch to control for contaminations by the laboratory equipment and environment.
Since personal care products of everyday use often contain parabens, the laboratory environment might be contaminated and this contamination might be transferred to the samples via the gloves worn by the experimenter during extraction of the plastic pieces and gel samples from the teethers. This source of contamination is not included in the procedural blanks and was thus investigated separately by analysis of the respective gloves. Therefore, one glove of the pair worn during laboratory work was compared to an unworn glove. For this, of each glove, four pieces, two of the fingertip from the index finger (one from each side of the glove, top and bottom) and two from the palm (top and bottom) were cut and subjected to UAE. Each piece had an area of approximately 4 cm2. The weight of all four pieces of the worn and unworn gloves was 0.32 and 0.30 g, respectively.
All samples for method validation were treated as the real samples described in the section Sample preparation and analyzed as single measurements.
Results and discussion
Identification of parabens and sorbic acid
Mass spectra and determined LRI values of BSTFA derivatives of MeP, EtP, n-PrP and sorbic acid in the samples showed good correlation with the derivatized reference substances. LRI on ZB-5 of trimethylsilylated MeP (MeP-d4) was 1494 (1495), of trimethylsilylated EtP 1568, of trimethylsilylated n-PrP 1667, and of trimethylsilylated sorbic acid 1183. Moreover, the determined LRI value of derivatized MeP also showed a good correlation with available NIST data (LRI reference value 1504). For LRI values of EtP, n-PrP and sorbic acid trimethylsilylesters no NIST or literature data were available. Good matches could also be achieved by comparison of the mass spectra of BSTFA derivatives of MeP, n-PrP and sorbic acid in the samples with available reference mass spectra of the NIST library with match factors of ≥850 (direct match). The mass spectrum of silylated EtP was not available in the NIST library.Method calibration
Initially, based on a seven-point calibration curve from 5 to 200 μg 10 mL−1 for each paraben, good linearity could be achieved in the concentration range of 10 to 200 μg 10 mL−1. This concentration range corresponds to approximately 0.05 to 0.91 ng absolute amount of each paraben on the GC separation column, under consideration of the applied split flow during sample injection. For each calibration level, the difference between the theoretical and calculated concentration was lower than 5%. Calibration curve data, with LOD and LOQ values of MeP, EtP, and n-PrP, are summarized in Table 1.| Compound | R 2 | LOD (μg 10 mL−1) | LOQ (μg 10 mL−1) |
|---|---|---|---|
| a Six calibration points; n = 1; calibration range 10–200 μg 10 mL−1; area ratio between the quantifier ion of the unlabeled compound and MeP-d4 was plotted against concentration (y = area ratio; x = concentration); LOD: limit of detection; LOQ: limit of quantification. | |||
| MeP | 0.9999 | 2.9 | 10.9 |
| EtP | 0.9998 | 4.3 | 16.1 |
| n-PrP | 0.9997 | 5.1 | 19.2 |
Method validation
The pre-concentrated solvents methanol and dichloromethane did not show any signals of silylated MeP, EtP, and n-PrP or other parabens, or sorbic acid. Procedural blanks could also prove that the laboratory equipment was free of paraben compounds and sorbic acid and that, therefore, no risk of cross-contamination of real samples was present. Nitrile gloves (worn and unworn) analyzed as additional control to assess the laboratory environment were also free of parabens and sorbic acid.Ideally, the internal standard MeP-d4 for quantification should be added to the sample material prior to all steps of the sample preparation method to balance possible extraction effects, particularly an incomplete extraction. However, in the case of the analysis of plastic material, MeP-d4 could not be introduced homogenously into the plastic samples with an appropriate method without possible negative effects on the original sample, e.g. losses of the target compounds. Additionally, some plastic and also gel sample extracts had to be diluted after UAE for the SIDA analysis. Thus, for the analyzed plastic and gel samples, the methanolic solutions were spiked with MeP-d4 only before evaporation and subsequent silylation of the target compounds with BSTFA.
For the reason that MeP-d4 could not be introduced into the plastic samples at the beginning of the sample preparation, it was also not possible to do real recovery experiments with the plastic material, and thus recovery could be performed only with the gel material. To calculate the recovery, a set of five independently spiked gel samples was analyzed (Table 2). Good recoveries of MeP, EtP and n-PrP could be achieved within the calibration range of 10 to 200 μg 10 mL−1, which ranged from 82 to 119%. Relative standard deviations (RSDs) of MeP, EtP, and n-PrP were lower than 15%. Recovery results show that with increasing alkyl chain-length the recovery values decrease. This could indicate that the isotope-labeled internal standard MeP-d4, which is also used for EtP and n-PrP, cannot compensate all effects occurring during the applied sample preparation and analysis method in the case of longer-chain paraben compounds, e.g. different silylation ratios of internal standard and analyte. For this reason, it is preferable to use EtP-d4 and n-PrP-d4 as isotope-labeled internal standards for quantification of EtP and n-PrP, respectively.
| Compound | Recovery at low calibration level (mean ± SD; %) | Recovery at medium calibration level (mean ± SD; %) | Recovery at high calibration level (mean ± SD; %) |
|---|---|---|---|
| a Calculated from five independently spiked samples. Recoveries at low (approx. LOQ), medium (approx. 100 μg 10 mL−1) and high (approx. 200 μg 10 mL−1) calibration levels were performed by dilution of the spiked samples. | |||
| MeP | 119 ± 5.3 | 106 ± 11.3 | 102 ± 8.1 |
| EtP | 109 ± 7.5 | 93 ± 7.1 | 82 ± 2.2 |
| n-PrP | 84 ± 11.6 | 82 ± 14.8 | 85 ± 12.2 |
Due to good recoveries no optimization of the applied basic analytical procedure was performed. However, optimization in regard to expense of material and time might be possible.
Repeatability measurements of MeP, EtP, and n-PrP in plastic and gel samples showed good analytical precisions with RSD smaller than 10% (Table 3).
| Compound | Plastic material (RSD; %) | Gel material (RSD; %) |
|---|---|---|
| a Repeatability expressed as relative standard deviation (RSD). Intra-day precision calculated from five independently prepared samples analyzed within one day. Inter-day precision calculated from five independently prepared samples analyzed on three days within one week. | ||
| Intra-day precision | ||
| MeP | 4.6 | 7.7 |
| EtP | 2.7 | 5.6 |
| n-PrP | 3.3 | 9.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Inter-day precision | ||
| MeP | 5.7 | 4.1 |
| EtP | 7.4 | 6.1 |
| n-PrP | 5.6 | 7.5 |
Paraben concentrations in gel-filled baby teethers
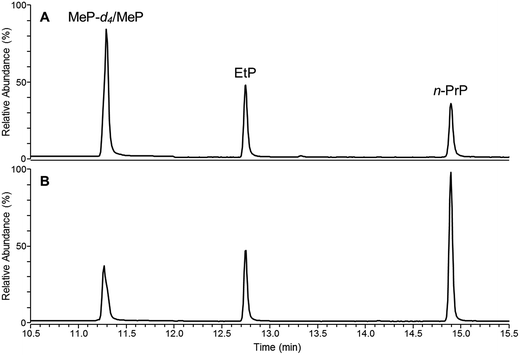
Results of plastic and gel material analysis to monitor the presence of paraben preservatives in the baby teethers are summarized in Table 4. The mean concentrations were calculated from single measurements of two independently prepared samples. The results demonstrate that all analyzed samples contain parabens in the plastic and the gel material. The fact that parabens can be found in the plastic material may explain the particular smell noticed while removing the baby teethers from their packaging, which was similar to the odor of the reference materials MeP and EtP. Fig. 1 shows a GC-qMS chromatogram of the calibration level 50 μg 10 mL−1 and of the gel sample of baby teether no. 3 in SIM mode (target screening).| Baby teether | Individual paraben concentrations (mean; μg g−1) | Total paraben amount (mean; mg g−1) | ||
|---|---|---|---|---|
| MeP | EtP | n-PrP | ||
| a Mean concentration value with range (number in brackets) from two independently prepared samples; LOD: limit of detection; LOQ: limit of quantification; SD: standard deviation. b Mean value and standard deviation (number in brackets) from repeatability test (intra-day precision, n = 5). c Same product as no. 1 but different lot number purchased about one year later. | ||||
| No. 1 plastic | 363 (16.6)b | <LOD | 382 (12.8)b | 0.74 |
| No. 1 gel | 930 (10.8) | <LOD | 31 (0.9) | 0.96 |
| No. 2c plastic | <LOD | 919 (15.0) | 249 (10.6) | 1.17 |
| No. 2c gel | <LOD | 241 (2.5) | 40 (3.8) | 0.28 |
| No. 3 plastic | 16 (0.5) | 261 (7.1)b | 836 (2.5) | 1.11 |
| No. 3 gel | 64 (4.9)b | 213 (11.8)b | 492 (45.3)b | 0.77 |
| No. 4 plastic | 344 (2.1) | <LOD | 179 (4.3) | 0.52 |
| No. 4 gel | 654 (5.8) | <LOD | <LOQ | 0.65 |
| No. 5 plastic | 274 (13.7) | <LOD | 38 (2.8) | 0.31 |
| No. 5 gel | 528 (20.3) | <LOD | <LOQ | 0.53 |
Interestingly, the results show some trends in distribution of parabens between plastic and gel material. With increasing alkyl chain-length paraben compounds are present in higher concentrations in the plastic material, while MeP values were higher in the gel material. Assuming that all parabens were added to the same component, the concentration differences between plastic and gel might be caused by diffusion of the analytes between both components. The concentration variations of the three investigated parabens in gel and plastic, respectively, are due to different polarities and the resulting solubilities in both components. However, swelling effects by influx of the gel of unknown composition into the plastic material EVA might also happen and would increase the transport of the parabens from the incorporated gel material into the plastic material.
Furthermore, in all gel samples sorbic acid could be identified. Like MeP and EtP and their sodium salts (E 214–219), also sorbic acid and its salts (E 200–203) are additives permitted in the EU for preservation of foods by inhibition of microbial growth.30 The use of these food additives is restricted by the regulation (EU) No. 1129/2011 (ref. 10) of the European Parliament and Council.
Results for baby teether no. 1 and 2 differed, although both were essentially the same product. They were, however, purchased in different years and had different batch numbers. Obviously EtP was used instead of MeP for baby teether no. 2, because only traces of MeP could be detected in the respective gel materiel (<LOD), confirming results of the non-target chemical analysis of the plastic material in the previous study.11
It remains unknown whether the parabens in the gel and plastic materials were added as a mixture to only one of them during the teether production and transferred into the other one by pure migration. Alternatively, the parabens might have been added as a mixture to both materials or one paraben might have been added to the gel and another paraben to the plastic. Apart from this, the microbiological stability of the gel is probably much more critical than that of the plastic. However, it is conceivable to incorporate such preservatives also into plastic polymers, like it is done in active packaging materials for food applications.31 In the case of baby teethers, a possible purpose for the application of preservatives would be to protect the chewing surface of EVA against microbial growth, since a baby teether will be intensively touched by the hands and moistened with saliva. One approach to investigate into which material the parabens were originally incorporated would be the analysis of newly produced baby teethers, since in this case both materials would have had a short contact time and probably migration effects would be still negligible at this time.
In the previous study,11 a migration test with baby teether no. 1 had shown that MeP and n-PrP migrated from the plastic material into water, however, no quantitative analysis was applied. To assess the risk from parabens in baby teethers to infants, quantification after a migration test with artificial saliva would be required. Nevertheless, the confirmed migration into water, which is rather similar to saliva, already shows the relevance of paraben contents in baby teethers. In the present study sample preparation was conducted with methanol to get a picture of the worst-case situation. These quantitative results need to be discussed in regard to present limit values in order to assess a potential health risk.
In the EU, the regulation (EC) No. 1223/2009 (ref. 32) supplemented by the regulation (EU) No. 358/2014 and No. 1004/2014 (ref. 9 and 33) establishes limit values for the preservation of cosmetic products. It is permitted to use MeP and EtP and their salts with a maximum concentration of 0.4% for each individual alkyl ester compound and n-PrP and n-butylparaben (n-BuP) and their salts with a maximum concentration of 0.14% for the sum of each individual alkyl ester compound (w/w, both calculated as acid). For mixtures, a total maximum concentration of 0.8% (w/w, calculated as acid) is permitted if the sum of n-PrP and n-BuP and their salts does not exceed 0.14%. If calculating the total amount of determined paraben concentrations in plastic and gel material of the analyzed baby teethers, respectively, values are considerably lower than the limit value of 0.8%, ranging from 0.03 to 0.12% in plastic and from 0.03 to 0.10% in gel material. However, independent of actual concentrations, it has also to be taken into account that the different exposure routes via the skin or via ingestion will result in different bioavailabilities of parabens.
The European Food Safety Authority (EFSA) published in 2004 (ref. 34) a temporary Acceptable Daily Intake (ADI) value of 10 mg kg−1 bw (bodyweight), as the sum of MeP and EtP and their sodium salts in foods. Since ADI values are normally related to adults, it can be assumed that an ADI for infants and young children would be lower. Due to its more severe effects on sex hormones and the male reproductive organs in juvenile rats, n-PrP was excluded from this group ADI. Calculating the total amount of paraben concentrations, even including n-PrP, in the whole plastic material of the chewing part of each product, the values are below this ADI value of 10 mg kg−1 bw d−1 (see Table 5). Bodyweights of 6.4 kg for girls and 7 kg for boys at the age of 4 months35–37 were taken into account, since the manufacturer labeled the product packaging with a recommendation that the use is suitable from the age of 4 months on. The calculated values are however likely to be overestimates of the real intake of parabens, since the extraction of the compounds by saliva during mastication is most probably less efficient than the methanol extraction applied in the present study. On the other side it is important to consider that if parabens leach from the plastic material during mastication, a continuous migration of parabens from the gel material into the plastic material should take place. This could at least theoretically lead to concentrations of leaching parabens that might be higher than concentrations resulting from the preparation of plastic material separated from the gel core. Moreover, the applied extraction of the plastic material might not have been total, which would also result in an underestimation of the paraben concentration.
| Baby teether | Amount of material (g) | Paraben amounta (mean; mg) | Percentage (%) of ADIb | |
|---|---|---|---|---|
| Girls | Boys | |||
| a Including n-PrP. b Calculated under the assumption of bodyweights of 6.4 kg for girls and 7 kg for boys at the age of 4 months.35–37 c Estimated paraben amount in gel of a freshly produced baby teether in the case that the preservatives were first added to the gel only and subsequently migrated into the plastic, i.e. paraben amount calculated as the sum of present paraben amount in plastic and gel material. | ||||
| No. 1 plastic | 24.4 | 18.2 | 28.4 | 26.0 |
| No. 1 gel | 43.1 | 41.5 | 64.8 | 59.2 |
| No. 1 total | 67.5 | 59.7c | 93.2 | 85.2 |
| No. 2 plastic | 26.1 | 30.4 | 47.6 | 43.5 |
| No. 2 gel | 46.7 | 13.1 | 20.5 | 18.7 |
| No. 2 total | 72.8 | 43.5c | 68.1 | 62.2 |
| No. 3 plastic | 17.5 | 19.4 | 30.3 | 27.7 |
| No. 3 gel | 42.1 | 32.3 | 50.5 | 46.2 |
| No. 3 total | 59.6 | 51.7c | 80.8 | 73.9 |
| No. 4 plastic | 8.6 | 4.5 | 7.0 | 6.4 |
| No. 4 gel | 6.4 | 4.2 | 6.5 | 6.0 |
| No. 4 total | 15.0 | 8.7c | 13.5 | 12.4 |
| No. 5 plastic | 13.8 | 4.3 | 6.7 | 6.2 |
| No. 5 gel | 12.4 | 6.6 | 10.3 | 9.4 |
| No. 5 total | 26.2 | 10.9c | 17.0 | 15.5 |
In the case of a damaged baby teether, the gel filling could also be ingested. In a worst-case situation, if all gel material would be ingested, the ADI value would still not be exceeded based on the determined paraben concentrations at the time of analyses of each product (see Table 5). Even if the total amount of parabens in plastic and gel material were taken into account (because this might be the amount present in the gel directly after production in case the preservatives were added to the gel only and migrated into the plastic) the ADI would not be reached (see Table 5). Independent of these calculations, the possible continuous migration of parabens between the plastic and gel material and the possible loss of parabens by migration into the packaging material of the product have to be considered as additional effects.
The regulation (EU) No. 1129/2011 (ref. 10) permits the use of MeP and EtP and their sodium salts as well as sorbic acid and its salts as additives at defined maximum concentrations in different foods. For certain foods it is also allowed to use these compounds according to the quantum satis principle (use as much as reasonably needed). However, it should be noted that MeP, EtP and n-PrP and their salts and sorbic acid and its salts are not listed as permitted preservatives of foods for infants and young children,10 and therefore their use is prohibited here. In the case of sorbic acid, the direct use in gel material of the considered baby teethers is less of a concern, since the presence in plastic material could not be confirmed.
Finally the question arises whether the findings of the present study concerning gel-filled baby teethers from one single manufacturer are representative of similar products from other manufacturers. Other products might not even contain different concentrations of preservatives (or even none at all), but might consist of a different basic plastic material, which could lead to an altered migration behavior for parabens.
Conclusion
The present study investigated if paraben compounds were used for the preservation of gel material with unknown composition that was the filling material in baby teethers of one single manufacturer. Furthermore, the basic plastic material of the teethers, an EVA copolymer, was analyzed for parabens. The main focus was to establish an accurate and reliable analytical method to quantify MeP, EtP, and n-PrP in gel and plastic materials. The described application of UAE using methanol as the extraction solvent, and the quantitative analysis of BSTFA derivatized parabens by GC-qMS, combined with SIDA, revealed good recoveries in gel material, which ranged from 82 to 119%. Thus, the analytical procedure with a SIDA approach is suitable for analysis of gel material. However, recovery results of n-PrP might be improved by using n-PrP-d4 as the isotope-labeled internal standard. Repeatability in plastic and gel material showed good analytical precisions with RSDs smaller than 10%. Recovery in the plastic material could not be performed, due to the lack of an adequate method to introduce the target compounds into the plastic samples.The presented analyses confirmed the results of a previous study11 and also the initial assumption of the present study that parabens were used for preservation in all analyzed products. Additionally, sorbic acid was identified as a further preservative in the gel material of the baby teethers.
At the moment there are no legal restrictions for the use of parabens in baby teether gel fillings or even in the plastic material. However, manufactures should ensure that no migration of these known endocrine disrupting compounds into human saliva could be possible. The determined concentrations of parabens in baby teethers were lower than the respective ADI for adults.
Nevertheless, the stringent requirements on the use of preservatives as food additives given by the regulation (EU) No. 1129/2011 (ref. 10) do not allow the addition of parabens to products used by the sensitive group of infants and young children. If manufactures do not intend to renounce with the use of parabens in baby teethers, particular attention and responsibility are required to avoid an oral uptake of these preservatives by infants and young children. Further studies with more products of different compositions and from different manufacturers have to be conducted to assess the overall hazard from this source.
References
- A. M. Peck, Anal. Bioanal. Chem., 2006, 386, 907–939 CrossRef CAS PubMed.
- M. G. Soni, I. G. Carabin and G. A. Burdock, Food Chem. Toxicol., 2005, 43, 985–1015 CrossRef CAS PubMed.
- T. Sabalitschka, Arch. Pharm., 1930, 268, 653–673 CrossRef CAS.
- E. J. Routledge, J. Parker, J. Odum, J. Ashby and J. P. Sumpter, Toxicol. Appl. Pharmacol., 1998, 153, 12–19 CrossRef CAS PubMed.
- J. R. Byford, L. E. Shaw, M. G. B. Drew, G. S. Pope, M. J. Sauer and P. D. Darbre, J. Steroid Biochem., 2002, 80, 49–60 CrossRef CAS PubMed.
- J. Boberg, C. Taxvig, S. Christiansen and U. Hass, Reprod. Toxicol., 2010, 30, 301–312 CrossRef CAS PubMed.
- Scientific Committee on Consumer Safety, 2010, SCCS/1348/10, Revision 22 March 2011.
- Scientific Committee on Consumer Safety, 2011, SCCS/1446/11.
- European Parliament and Council, Official Journal of the European Union, 2014, No. 358/2014.
- European Parliament and Council, Official Journal of the European Union, 2011, No. 1129/2011.
- E. Berger, T. Potouridis, A. Haeger, W. Püttmann and M. Wagner, J. Appl. Toxicol., 2015, 35, 1254–1261 CrossRef CAS PubMed.
- P. E. Mahuzier, K. D. Altria and B. J. Clark, J. Chromatogr. A, 2001, 924, 465–470 CrossRef CAS PubMed.
- M. Jaworska, Z. Szulinska and M. Wilk, J. Sep. Sci., 2005, 28, 137–143 CrossRef CAS PubMed.
- S. P. Wang and C. L. Chang, Anal. Chim. Acta, 1998, 377, 85–93 CrossRef CAS.
- L. Labat, E. Kummer, P. Dallet and J. P. Dubost, J. Pharm. Biomed. Anal., 2000, 23, 763–769 CrossRef CAS PubMed.
- T. J. Yang, F. J. Tsai, C. Y. Chen, T. C. C. Yang and M. R. Lee, Anal. Chim. Acta, 2010, 668, 188–194 CrossRef CAS PubMed.
- A. Zotou, I. Sakla and P. D. Tzanavaras, J. Pharm. Biomed. Anal., 2010, 53, 785–789 CrossRef CAS PubMed.
- A. Prichodko, M. Mockunaite, V. Smitiene and V. Vickackaite, Chemija, 2011, 22, 155–161 CAS.
- L. Sanchez-Prado, G. Alvarez-Rivera, J. P. Lamas, M. Lores, C. Garcia-Jares and M. Llompart, Anal. Bioanal. Chem., 2011, 401, 3293–3304 CrossRef CAS PubMed.
- M. Gonzalez, M. Gallego and M. Valcarcel, J. Chromatogr. A, 1998, 823, 321–329 CrossRef CAS.
- M. Gonzalez, M. Gallego and M. Valcarcel, J. Chromatogr. A, 1999, 848, 529–536 CrossRef CAS PubMed.
- B. Saad, M. F. Bari, M. I. Saleh, K. Ahmad and M. K. M. Talib, J. Chromatogr. A, 2005, 1073, 393–397 CrossRef CAS PubMed.
- C. Milo and I. Blank, ACS Symp. Ser., 1998, 705, 250–259 CrossRef CAS.
- M. Rychlik and S. Asam, Anal. Bioanal. Chem., 2008, 390, 617–628 CrossRef CAS PubMed.
- M. Rychlik and S. Asam, Umweltwiss. Schadst.–Forsch., 2009, 21, 470–482 CrossRef CAS.
- J. Drozd, J. Chromatogr., 1975, 113, 303–356 CrossRef CAS.
- B. D. Zellner, C. Bicchi, P. Dugo, P. Rubiolo, G. Dugo and L. Mondello, Flavour Fragrance J., 2008, 23, 297–314 CrossRef.
- H. van Den Dool and P. D. Kratz, J. Chromatogr. A, 1963, 11, 463–471 CrossRef CAS.
- Chemical analysis - Decision limit, detection limit and determination limit under repeatability conditions - Terms, methods, evaluation, DIN 32645, Deutsches Institut für Normung, Beuth Verlag, Berlin, 2008 Search PubMed.
- J. D. Stopforth, J. N. Sofos and F. F. Busta, in Antimicrobials in food, ed. P. M. Davidson, J. N. Sofos and A. L. Branen, CRC Press, Boca Raton, 3rd edn, 2005, ch. 3, pp. 49–90 Search PubMed.
- P. Appendini and J. H. Hotchkiss, Innovative Food Sci. Emerging Technol., 2002, 3, 113–126 CrossRef CAS.
- European Parliament and Council, Official Journal of the European Union, 2009, No. 1223/2009.
- European Parliament and Council, Official Journal of the European Union, 2014, No. 1004/2014.
- The European Food Safety Authority, EFSA J., 2004, 83, 1–26 Search PubMed.
- M. de Onis, R. Martorell, C. Garza and A. Lartey, Acta Paediatr., 2006, 95, 76–85 Search PubMed.
- WHO Multicentre Growth Reference Study Group, Geneva: World Health Organization, 2006, http://www.who.int/childgrowth/standards/en/, accessed May 29 2015.
- Centers for Disease Control and Prevention, CDC, Atlanta, 2010, http://www.cdc.gov/growthcharts/who_charts.htm, accessed May 29 2015.
| This journal is © The Royal Society of Chemistry 2016 |