Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The potential for complementary targeted/non-targeted screening of novel psychoactive substances in equine urine using liquid chromatography-high resolution accurate mass spectrometry
Adam
Cawley
*a,
Daniel
Pasin
b,
Namuun
Ganbat
b,
Laura
Ennis
a,
Corrine
Smart
a,
Candace
Greer
a,
John
Keledjian
a,
Shanlin
Fu
b and
Alex
Chen
c
aAustralian Racing Forensic Laboratory, Racing NSW, Level 11, 51 Druitt Street, Sydney, NSW 2000, Australia. E-mail: acawley@racingnsw.com.au; Fax: +61 2 95517712; Tel: +61 2 83445000
bCentre for Forensic Science, University of Technology Sydney, NSW, Australia
cThermo Fisher Scientific, Scoresby, VIC, Australia
First published on 25th January 2016
Abstract
The potential for liquid chromatography-high resolution accurate mass (LC-HRAM) spectrometry to identify ‘unknown’ compounds using non-targeted screening methods provides a potential advantage in the fight against doping in sport. This innovation comes with the requirement for assessment to support its use in the medico-legal context. A method for the LC-HRAM detection of 2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (NBOMe) compounds in equine urine was validated in order to assess the capabilities of a workflow developed for non-targeted analysis using the SIEVE® differential analysis software platform. Six NBOMe compounds (25B, 25C, 25D, 25E, 25H and 25I) were studied to develop and optimize the proposed non-targeted screening workflow before two additional candidates (25N and 25T2) were used as blind controls for verification. Chromatographic alignment and the integration threshold were found to be the most critical parameters for successful identification of ‘unknown’ responses. The proposed workflow serves as an example for anti-doping laboratories to implement fit-for-purpose non-targeted screening methods.
Introduction
Novel psychoactive substances (NPS) are chemical modifications of currently controlled substances that have similar pharmacological effects and chemically designed to circumvent legislation.1 In 2012, Casale and Hays2 reported the characterization of eleven 2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (NBOMe) compounds considered to be more potent serotonin 5-HT2A receptor agonists than their 2,5-dimethoxyphenethylamine (“2C”) precursors.3–5 Since this time there have been multiple reports concerning adverse health effects.6–13 Misuse of this drug class generally occurs with a single administration of approximately 0.1 g to achieve hallucinations and a varying degree of stimulation.13 In response to a medico-legal requirement the validation of analytical methods used to detect NBOMe compounds in biological matrices has been reported by forensic toxicology laboratories.8–10 Furthermore, metabolism studies in human and rat urine have been recently performed.14While there are no reports describing the effect of these compounds in horses, there is concern about the potential for misuse of NPS such as NBOMe compounds in equine athletes where handlers may have made the assumption that these compounds are undetectable by horseracing laboratories. The availability of these compounds presents a serious threat to the integrity of equine sports and to the welfare of the horse. NBOMe compounds are therefore prohibited for use by the International Federation of Horseracing Authorities.15
Analytical methods utilizing the sensitivity and specificity of liquid chromatography-high resolution accurate mass (LC-HRAM) spectrometry applied to forensic toxicology,16–18 and more specifically to horseracing laboratories,19 have been reported in recent years. The potential for LC-HRAM technology to detect ‘unknown’ compounds using non-targeted screening methods provides a potential advantage in the fight against doping in sport.
Differential analysis is a tool used in studies with large data sets that require the comparison of pre-treatment control samples of biological origin with samples collected following a treatment (such as exposure to particular stimuli) in order to detect and elucidate biomarkers correlating to the treatment response. The control and treatment samples are compared by statistical means resulting in the generation of a list of targets that are independent of the control sample. For the past 10 to 15 years, differential analysis techniques have been widely used for the assessment of microarray data.20,21 More recently following the development of software for MS-based applications, there is considerable potential for differential analysis in analytical chemistry.22 Depending on the software used standard outputs may include retention time, mass-to-charge (m/z) ratio and the statistical probability (p-value) of the two samples being statistically different. From this information, a targeted approach can be applied such as tandem mass spectrometry (MS2) to identify unknown compounds. This strategy is preferred over conventional visual assessment of chromatographic data since it can detect compounds that coelute or have low abundances obscured by background noise. In addition, differential analysis applied to full-scan MS provides an advantage over other chemometric approaches for biomarker detection by not excluding raw data. With the rapid proliferation of NPS such strategies have the potential to detect new compounds that have not been previously defined in published targeted methods or databases.
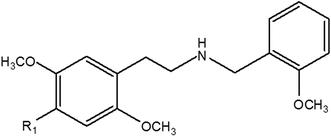
The aim of the study described herein was to develop and validate a method for the detection of six NBOMe compounds (Fig. 1) in equine urine using LC-HRAM spectrometry to support integrity in horseracing. Furthermore, the applicability of using a differential analysis software package such as SIEVE® (Statistical Iterative Exploratory Visualization Environment, Thermo Fisher Scientific)23 was evaluated for the non-targeted screening and putative identification of two additional NBOMe compounds that were unknown to the analyst under ‘blind’ testing conditions.
Materials and methods
Reference materials, chemicals and reagents
Hydrochloride salts of the NBOMe compounds [2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine] (25B), [2-(4-chloro-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine] (25C), [2-(4-methyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine] (25D), [2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine] (25E), [2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine] (25H), [2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine] (25I), [2-(4-nitro-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine] (25N) and [2-(4-methylthio-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine] (25T2) manufactured by Lipomed AG (Arlesheim, Switzerland) were purchased as 1 mg mL−1 (in methanol) ampoules from PM separations (Capalaba, Queensland, Australia). Desipramine-d3 was purchased from Grace (Deerfield, IL, USA). Trypsin and sodium acetate were obtained from Sigma-Aldrich (St Louis, MO, USA). β-Glucuronidase K12 from E. coli was purchased from Roche Diagnostics (Mannheim, Germany). Analytical grade ammonia (aqueous solution 28%) and acetic acid (glacial) together with HPLC grade solvents were purchased from Thermo Fisher Scientific (Fair Lawn, NJ, USA). Ultra-pure water was obtained using a Millipore filtration system (Bedford, MA, USA).Preparation of standard solutions
Stock solutions for each NBOMe standard were prepared at 100 μg mL−1 by quantitatively diluting the purchased 1 mg mL−1 solution into 10 mL of methanol in a volumetric flask. For the method validation, a mixed NBOMe intermediate solution (1 μg mL−1) containing the six candidates (25B, 25C, 25D, 25E, 25H and 25I) was prepared by diluting 100 μL of each stock into 10 mL of methanol in a volumetric flask. A mixed NBOMe working solution (100 ng mL−1) was prepared by diluting 1 mL of the mixed intermediate solution into 10 mL methanol in a volumetric flask. Desipramine-d3 stock solution (1 mg mL−1) was prepared from dissolving 5 mg of primary standard, weighed using an analytical balance (Mettler Toledo AT261, Columbus, OH, USA), in methanol (5 mL) using a volumetric flask. The desipramine-d3 working solution (2 μg mL−1) was prepared by diluting 20 μL of stock in methanol (10 mL) using a volumetric flask. Methanolic solutions were stored at 4 °C for up to 12 months.Preparation of blank equine urine
Authentic blank urine samples were collected from three thoroughbred gelding horses by spontaneous voiding with carrot reward following approval from the Racing NSW Animal Care and Ethics Committee (RP72). These horses were known to have not been administered any pharmaceutical agent for at least two weeks prior to sample collection. Urine samples from the three horses were pooled to provide a profile considered to be representative of the racehorse population for proof-of-concept concerning differential analysis.Sample preparation
Equine urine samples (3 mL) were fortified with desipramine-d3 (2 μg mL−1, 50 μL) internal standard before addition of pH 5.0 acetate buffer (0.2 M, 4 mL) followed by enzyme hydrolysis with β-glucuronidase K12 from E. coli (20 μL) and trypsin solution (625 μg per sample) overnight at 37 °C. The basic organic fraction was isolated by solid phase extraction (SPE) using a mixed-mode C8/strong cation exchange XTRACKT® column (200 mg, 3 cc, UCT, Bristol, PA, USA). The cartridge was conditioned with methanol (2 mL) and water (2 mL) before loading the urine sample and washing with acetic acid (0.1 M, 2 mL) followed by methanol (2 mL). The cartridge was dried using N2 gas under positive pressure before elution with ethyl acetate/ammonia/methanol (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 3 mL). HCl/methanol (0.1 M, 20 μL) was added to extracts before evaporation to dryness under nitrogen at 60 °C. Residues were reconstituted in methanol (50 μL) and pH 4 ammonium acetate (10 mM, 100 μL) for LC-HRAM analysis.
0.5, 3 mL). HCl/methanol (0.1 M, 20 μL) was added to extracts before evaporation to dryness under nitrogen at 60 °C. Residues were reconstituted in methanol (50 μL) and pH 4 ammonium acetate (10 mM, 100 μL) for LC-HRAM analysis.
LC-HRAM analysis
LC-HRAM spectrometry was undertaken using an Ultimate 3000 HPLC coupled to a QExactive benchtop orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). LC separation was performed using a Gemini® C18 column (50 × 2.1 mm, 5 μm; Phenomenex, Torrence, CA, USA) operating at 35 °C with a 10 μL injection volume. The mobile phase consisted of A: pH 9 ammonium acetate (10 mM) and B: 0.1% acetic acid/acetonitrile. Gradient elution was performed with a flow rate of 0.5 mL min−1 according to the following program: 1% B for 2 min, increased to 80% B linearly during the period between 2 and 8.5 min, before returning to 1% B at 8.6 min and held until 11.2 min. HRAM detection was performed using positive mode heated electrospray ionization (HESI) in full scan at a resolution of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (full width at half maximum, FWHM) acquiring a mass range of m/z 50 to 650 at 3 Hz. Mass calibration was performed prior to analysis using Pierce® ESI positive (P/N 88
000 (full width at half maximum, FWHM) acquiring a mass range of m/z 50 to 650 at 3 Hz. Mass calibration was performed prior to analysis using Pierce® ESI positive (P/N 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323) calibration solution (Thermo Fisher Scientific, Bremen, Germany) but no lock mass was used. Source temperature, spray voltage, sheath gas (high purity N2) and auxiliary gas (ultra-high purity N2) were set at 350 °C, +4000 V, 63.74 and 10.30 arbitrary units, respectively. MS2 data was acquired with a normalized collision energy of 25 arbitrary units, automatic gain control target of 2 × 10e5 and m/z isolation window of 0.5 to support confirmation of identity according to criteria prescribed by the Association of Official Racing Chemists (AORC).24 Instrument control and data processing (±5 ppm) were performed using Xcalibur® software (version 2.2 SP1) from Thermo Fisher Scientific (San Jose, CA, USA).
323) calibration solution (Thermo Fisher Scientific, Bremen, Germany) but no lock mass was used. Source temperature, spray voltage, sheath gas (high purity N2) and auxiliary gas (ultra-high purity N2) were set at 350 °C, +4000 V, 63.74 and 10.30 arbitrary units, respectively. MS2 data was acquired with a normalized collision energy of 25 arbitrary units, automatic gain control target of 2 × 10e5 and m/z isolation window of 0.5 to support confirmation of identity according to criteria prescribed by the Association of Official Racing Chemists (AORC).24 Instrument control and data processing (±5 ppm) were performed using Xcalibur® software (version 2.2 SP1) from Thermo Fisher Scientific (San Jose, CA, USA).
| NBOMe | Retention time (min) | Formula | Experimental [M + H]+ | Theoreticala [M + H]+ | Mass error (Δ ppm) |
|---|---|---|---|---|---|
| a Determined by isotope simulation tool in the Xcalibur® operating software. | |||||
| 25H | 5.72 | C18H23NO3 | 302.1745 | 302.1751 | 2.0 |
| 25B | 6.20 | C18H22NO3Br | 380.0850 | 380.0856 | 1.6 |
| 382.0829 | 382.0835 | 1.6 | |||
| 25C | 6.10 | C18H22NO3Cl | 336.1355 | 336.1361 | 1.8 |
| 338.1325 | 338.1331 | 1.8 | |||
| 25D | 6.10 | C19H25NO3 | 316.1902 | 316.1907 | 1.6 |
| 25E | 6.48 | C20H27NO3 | 330.2059 | 330.2064 | 1.5 |
| 25I | 6.37 | C18H22NO3I | 428.0712 | 428.0717 | 1.2 |
Analysis of MS2 data to investigate dissociation schemes and apply precursor ion fingerprinting was performed using Mass Frontier™ 7.0 SR1. Each of these software packages were purchased from Thermo Fisher Scientific (San Jose, CA, USA).
Results and discussion
Targeted method validation
The limited LC selectivity displayed by the six candidate NBOMe compounds is shown in Table 1 with retention times within 0.8 min, including coelution of 25C and 25D. Nevertheless, specificity was achieved from differences in mass for this group of non-isomeric NBOMe compounds. The mass error (Δ) for the six candidate NBOMe compounds was found to be within 2.0 ppm (Table 1), which is within the accepted value of 5.0 ppm.24 This included the [M + H + 2]+ isotopes for 25B and 25C due to the presence of bromine and chlorine atoms in their respective structures. Robustness in specificity was demonstrated by stability in mass accuracy without the use of a lock mass. Using caffeine ([M + H]+ = m/z 195.0877) as the most representative compound of NPS contained in the calibration mix, the intra-assay standard deviation was within 0.05 ppm. For the six-month duration of the study, the inter-assay standard deviation was within 0.1 ppm.Quantitative validation results are provided in Table 2, which shows the LOD to be 0.1 ng mL−1 for 25B, 25E, 25H and 25I, and 0.5 ng mL−1 for 25C and 25D. The LLOQ for all six candidate NBOMe compounds was 1 ng mL−1. Linearity was achieved between this LLOQ and 200 ng mL−1 with R2 ≥ 0.995 to establish the calibration range performed in duplicate at the following concentrations: 1, 2, 5, 10, 20, 50, 100 and 200 ng mL−1. Intra- and inter-assay precision was assessed at a spiking level of 1 ng mL−1 to be within 8.7% (<10%) and 13.2% (<15%), respectively. At 10 ng mL−1 these were within 4.0% (<10%) and 10.9% (<15%), respectively. Suitability of the SPE sample preparation method was demonstrated by excellent recoveries (88–97%) from 10 ng mL−1 spiked equine urine samples, supporting accuracy between 93 ± 4% and 113 ± 6% (between 80% and 120%). Matrix effects were determined to be negligible from the results that were within 20% of the expected value. Samples were stable after 3 freeze/thaw cycles and stable for up to 3 months at 4 °C, −20 °C and −80 °C.
| Parameter | 25B | 25C | 25D | 25E | 25H | 25I |
|---|---|---|---|---|---|---|
| LOD (ng mL−1) | 0.1 | 0.5 | 0.5 | 0.1 | 0.1 | 0.1 |
| LLOQ (ng mL−1) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Linearity (ng mL−1, R2 ≥ 0.995) | 1–200 | 1–200 | 1–200 | 1–200 | 1–200 | 1–200 |
| Intra-assay at 1 ng mL−1 (%RSD) | 6.9 | 8.5 | 7.3 | 6.2 | 8.7 | 5.7 |
| Intra-assay at 10 ng mL−1 (%RSD) | 5.2 | 5.4 | 6.1 | 4.0 | 7.3 | 3.5 |
| Inter-assay at 1 ng mL−1 (%RSD) | 6.7 | 7.5 | 10.5 | 13.2 | 11.8 | 8.6 |
| Inter-assay at 10 ng mL−1 (%RSD) | 5.3 | 7.6 | 5.9 | 5.0 | 10.9 | 6.2 |
| Accuracy (%±SD, at 10 ng mL−1) | 93 ± 4 | 109 ± 5 | 108 ± 5 | 106 ± 4 | 107 ± 7 | 113 ± 6 |
| Recovery (%, at 10 ng mL−1) | 93 | 97 | 96 | 97 | 91 | 88 |
Non-targeted analysis using differential analysis
Individual components of the SIEVE® software workflow were assessed; chromatographic alignment, peak detection, statistical analysis and identification.The default Peak Intensity Threshold setting of 1.27 × 108 analyzing 5000 frames provided detection of the six candidate NBOMe compounds at concentrations between 20 and 100 ng mL−1. While sufficient for proof-of-concept that differential analysis was applicable to the method, these levels were considered too high for routine application. Optimization of the Peak Intensity Threshold to 1 × 106 analyzing 2000 frames resulted in detection levels between 0.5 ng mL−1 and 10 ng mL−1 that are considered fit-for-purpose.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.90 and 1.00 × 10−5, which implies the compound in the treatment sample is completely independent to the control sample. In addition to numerical statistical evaluation, the data can be reviewed as volcano plots, scores plots from principal component analysis (PCA) or scatter plots to visualize correlation between Ratio and p-value.
999.90 and 1.00 × 10−5, which implies the compound in the treatment sample is completely independent to the control sample. In addition to numerical statistical evaluation, the data can be reviewed as volcano plots, scores plots from principal component analysis (PCA) or scatter plots to visualize correlation between Ratio and p-value.
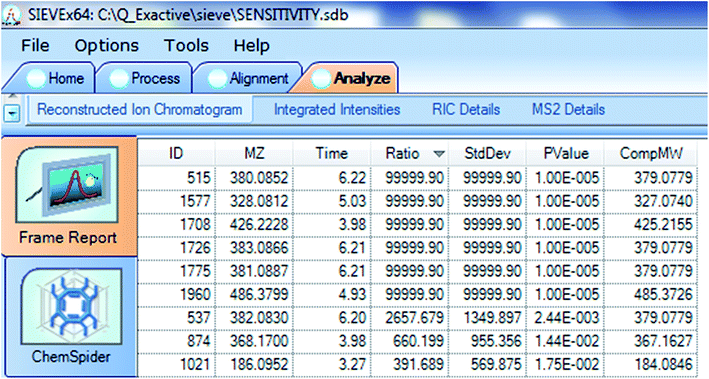
For this study, compounds exhibiting a Ratio > 10 and p-value < 0.001 were deemed compounds of interest. Fig. 3 shows the frame report for 25B spiked at 5 ng mL−1 with maximum Ratio values of 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.90 for m/z 380.0852 ([M + H]+, Δ = 1.1 ppm) and 2657.679 for m/z 382.0830 ([M + H + 2]+, Δ = 1.3 ppm), together with 99
999.90 for m/z 380.0852 ([M + H]+, Δ = 1.1 ppm) and 2657.679 for m/z 382.0830 ([M + H + 2]+, Δ = 1.3 ppm), together with 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.90 for their 13C isotope responses m/z 381.0887 and m/z 383.0866, respectively. The p-value associated with the maximum possible Ratio values was 1.00 × 10−5, while that for m/z 382.0830 with a lower yet clearly high Ratio value was 2.44 × 10−3. Interestingly, these m/z values only differ by approximately 1 Da yet they are all associated with different frames. For example m/z 380.0852, 381.0887, 382.0830 and 383.0866 are associated with frames 515, 1775, 537 and 1726 and respectively.
999.90 for their 13C isotope responses m/z 381.0887 and m/z 383.0866, respectively. The p-value associated with the maximum possible Ratio values was 1.00 × 10−5, while that for m/z 382.0830 with a lower yet clearly high Ratio value was 2.44 × 10−3. Interestingly, these m/z values only differ by approximately 1 Da yet they are all associated with different frames. For example m/z 380.0852, 381.0887, 382.0830 and 383.0866 are associated with frames 515, 1775, 537 and 1726 and respectively.
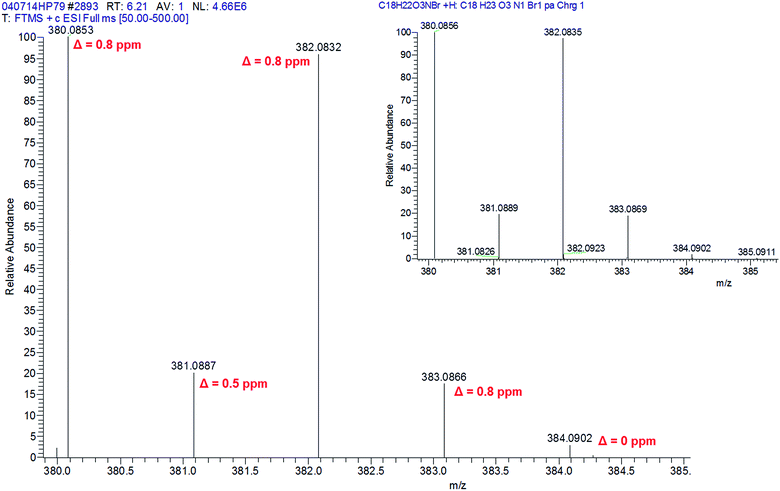 | ||
| Fig. 4 Xcalibur® simulated isotope pattern for 25B spiked at 5 ng mL−1 in equine urine (with annotated Δ) and theoretical pattern for C18H22O3NBr (right inset). | ||
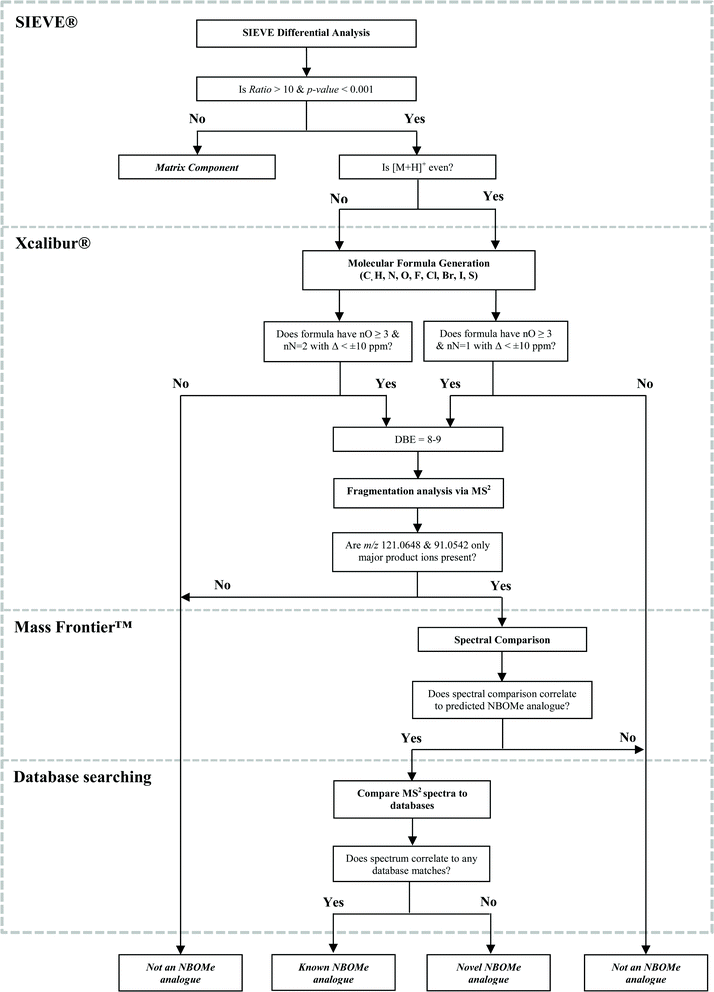
With the molecular weight and chemical formula proposed for an ‘unknown’ compound using SIEVE® and Xcalibur®, respectively, the identification process can proceed using MS2 studies. For this purpose Mass Frontier™ spectral interpretation software was used to interrogate targeted MS2 data uploaded from Xcalibur®. Mass Frontier™ software can predict product-ion structures and provide dissociation pathways for user-defined compounds. Precursor ion fingerprinting was investigated as a means for using structurally-related product ions to identify the precursor.29 MS2 studies of the six candidate NBOMe compounds revealed a common dissociation pathway resulting in the cleavage of benzyl moiety to produce m/z 121.0651 (±1.2 ppm) and detection of the tropylium ion (m/z 91.0548 (±1.8 ppm)). These diagnostic product ions can be used to confirm the NBOMe class of compounds following investigation of screening abnormalities produced from differential analysis.
It should be considered that while the strategy outlined here is aimed explicitly at users of Thermo Fisher Scientific hardware-software configurations for screening of NBOMe compounds, the general workflow can be adapted by users of instruments manufactured by other vendors. For instance, SIEVE® is not the only differential analysis software package available to LC-HRAM practitioners; Mass Profiler Professional developed by Agilent Technologies (Santa Clara, CA, USA) and MetaboLynx™ developed by Waters Corp. (Milford, MA, USA) are also available.
Test case 1. In conjunction with the tabulated frame report, a scatter plot of Ratio versus p-value is provided by SIEVE® for rapid visual inspection of differential analysis results. Selection of an outlier with a Ratio of 416.063 and p-value of 1.16 × 10−2 had a corresponding m/z entry with 347.1596 at a retention time of 6.07 min. In addition, a 13C isotopic response with a Ratio of 15.252 and p-value 1.01 × 10−2 was found with m/z 348.1631 at the same retention time and CompMW of 346.1524 Da. Simulated spectral comparison provided good agreement (Δ = 1.4 ppm and Δ = 1.1 ppm) between the two ions detected by SIEVE® and the theoretical m/z, respectively. While the p-value for both ions was relatively high (i.e. >1 × 10−3), the retention time alignment of these responses with high Ratio values was considered sufficient evidence for entry into Xcalibur® to determine the chemical formula as C18H22N2O5 with Δ = 0.2 ppm and DBE = 9.0. Mass Frontier™ analysis of MS2 data revealed m/z 91.0545 and m/z 121.0646 belonging to the diagnostic fragments of an NBOMe compound. The above information was used to identify the unknown compound as the nitro-NBOMe compound, 25N with comparison to literature data reported by Casale and Hays.2 The DBE value found in this case was 9.0 instead of 8.0 found for other NBOMe compounds, thereby providing a characteristic feature of the NO2-analogue.
Test case 2. A second analyst selected an outlier with a Ratio of 194.980 and p-value of 6.38 × 10−3 that had a corresponding m/z entry with 363.1814 at a retention time of 6.64 min. A second entry at 6.64 min with m/z 362.1779 had a Ratio of 86.715 and p-value of 9.43 × 10−4 with the CompMW for both entries at 361.1706 Da. Xcalibur® determined the chemical formula to be C20H27NO3S with Δ = −0.04 ppm and DBE = 8.0. The simulated spectral comparison revealed excellent agreement for m/z 362.1781 (100%, Δ = 0.8 ppm), m/z 363.1816 (20% rel., Δ = 0.6 ppm), m/z 364.1736 (5% rel., Δ = 1.6 ppm) and m/z 365.1771 (1% rel., Δ = 1.4 ppm). Precursor ion fingerprinting of MS2 data confirmed the NBOMe class before web searching of the chemical formula provided the putative identification to be 25T2.30,31
Illicit administration of NPS such as NBOMe compounds to racehorses is highly possible in response to surveillance of conventional amphetamine-type substances by laboratories. This requires accredited racing laboratories to implement testing methods for substances that become readily available on the black market in order to deter and prosecute licensed persons who are tempted to administer non-approved drugs without knowledge of adverse health effects to an animal. Since 2013, the Australian Racing Forensic Laboratory (ARFL) has no reported findings for NBOMe compounds in equine urine.
The challenge of screening for an ever-increasing number of target compounds, many of which may be ‘unknown’ to a laboratory or for which no reference material is available, necessitates the use of hardware-software technology combinations that can assist in identifying abnormal responses in a biological sample for further investigation. To this end, the advantages of HRAM instrumentation in racing laboratories can be further enhanced by incorporating differential analysis software such as SIEVE® investigated in this study.
The individual components of the SIEVE® software workflow were assessed to identify critical control points that could be optimized for a proposed non-targeted analysis workflow in forensic laboratories. The importance of mass accuracy for confidence in determining unknowns should not be understated. Following the assessment of this parameter to document specificity in method validation, successful differential analysis was shown by this study to be dependent on robustness of chromatographic alignment and optimization of the Peak Intensity Threshold to achieve the required sensitivity. Recursive-base-peak-framing provides retention time, m/z, Ratio, p-value and CompMW that can be reviewed to determine an abnormal finding. While the processing time (approximately 1 min) for differential analysis per sample is reasonable, further work is required to enable batch processing of multiple sample files for more efficient translation of this workflow to routine testing. Regardless, the proposed workflow was used by the ARFL in conjunction with routine targeted analysis of 95 equine urine samples over a three-week period during a major racing carnival. No abnormalities requiring further investigation have yet been reported, however work continues to increase the coverage of samples analyzed by this method.
The CompMW can be used by the instrument operating software (Xcalibur®) to provide a putative chemical formula within defined parameters for elemental composition, which is critical to ensure sufficient coverage of NPS that may include halogen and sulfur substituents. In addition, DBE was found to be a useful discriminator of NPS classes, with 8 or 9 (in the case of 25N) being characteristic of NBOMe compounds. Subsequent MS2 data was interrogated using Mass Frontier™ to enable precursor ion fingerprinting where diagnostic product ions could identify the NBOMe present. The major limitation of this study was the reliance on conventional literature sources to facilitate the final stage of compound identification from MS2 data. Further work is required to investigate the possibility that pseudo-MS3 (using in-source collision-induced dissociation, CID) studies of small molecules such as NBOMe compounds could provide the means for in silico structural elucidation. This would allow collaborative approaches such as mzCloud,32 a MSn spectral database, to be incorporated into the workflow.
An additional limitation is the focus on parent NBOMe compounds without taking into account the likely presence of metabolites following NBOMe administration as recently reported for 25I by Caspar et al.14 Metabolism of NBOMe and other NPS compounds will undoubtedly be important to consider as the research into non-targeted screening strategies continues, however the focus of this study was to use parent NBOMe compounds as model analytes for the development of non-targeted screening strategies.
Consideration must also be given to the presence of isobaric compounds such as structural isomers that are not resolved using HRAM technology. Investigation and subsequent confirmation of such compounds will require greater LC selectivity than the generic screen presented here.
Notwithstanding the limitations of the method, putative identification of a NBOMe compound using the presented screening strategy can enable an abnormality to be recorded against a sample in order for further investigation to take place. Subsequent confirmation would require the procurement of an authentic reference material but this should not preclude the method from being useful to circumvent the nexus that anti-doping laboratories find themselves in terms of targeted analysis.
Conclusion
The use of LC-HRAM spectrometry has demonstrated the capability for laboratories to combat the misuse of NPS in horseracing. Specific to this study, the presented method for the detection and quantification of NBOMe compounds in equine urine was validated. In addition, proof-of-concept use of differential analysis with SIEVE® was performed for non-targeted screening to putatively identify two NBOMe compounds, 25N and 25T2 with scope to extend this study to MSn data for in silico elucidation of novel compounds.References
- M. Collins, Drug Test. Anal., 2011, 3, 404–416 CrossRef CAS PubMed.
- J. F. Casale and P. A. Hays, Microgram, 2012, 9, 84–109 CAS.
- D. Zuba and K. Sekula, Drug Test. Anal., 2013, 5, 634–645 CrossRef CAS PubMed.
- K. Sekula and D. Zuba, Rapid Commun. Mass Spectrom., 2013, 27, 2081–2090 CrossRef CAS PubMed.
- D. Zuba, K. Sekula and A. Buczek, Forensic Sci. Int., 2013, 227, 7–14 CrossRef CAS PubMed.
- S. Rutherfoord Rose, J. L. Poklis and A. Poklis, Clin. Toxicol., 2013, 51, 174–177 CrossRef PubMed.
- S. L. Hill, T. Doris, S. Gurung, S. Katebe, A. Lomas, M. Dunn, P. Blain and S. H. L. Thomas, Clin. Toxicol., 2013, 51, 487–492 CrossRef CAS PubMed.
- J. L. Poklis, C. R. Nanco, M. M. Troendle, C. E. Wolf and A. Poklis, Drug Test. Anal., 2014, 6, 764–769 CrossRef CAS PubMed.
- J. L. Poklis, D. J. Clay and A. Poklis, J. Anal. Toxicol., 2014, 38, 113–121 CrossRef CAS PubMed.
- R. D. Johnson, S. R. Botch-Jones, T. Flowers and C. A. Lewis, J. Anal. Toxicol., 2014, 38, 479–484 CrossRef PubMed.
- S. J. Stellpflug, S. E. Kealey, C. B. Hegarty and G. C. Janis, J. Med. Toxicol., 2014, 10, 45–50 CrossRef CAS PubMed.
- M. H. Y Tang, C. K. Ching, M. S. H. Tsui, F. K. C. Chu and T. W. L. Mak, Clin. Toxicol., 2014, 52, 561–565 CrossRef PubMed.
- M. F. Weaver, J. A. Hopper and E. W. Gunderson, Addiction Science & Clinical Practice, 2015, vol. 10, pp. 1–9 Search PubMed.
- A. T. Caspar, A. G. Helfer, J. A. Michely, V. Auwãrter, S. D. Brandt, M. R. Meyer and H. H. Maurer, Anal. Bioanal. Chem., 2015, 407, 6697–6719 CrossRef CAS PubMed.
- International Federation of Horseracing Authorities. Article 6 of the International Agreement on Breeding, Racing and Wagering, http://www.ifhaonline.org/agreementDisplay.asp#a6, accessed May 25th 2015.
- I. Ojanperã, M. Kolmonen and A. Pelander, Anal. Bioanal. Chem., 2012, 403, 1203–1220 CrossRef PubMed.
- M. R. Meyer and H. H. Maurer, Anal. Bioanal. Chem., 2012, 403, 1221–1231 CrossRef CAS PubMed.
- M. R. Meyer, A. G. Helfer and H. H. Maurer, Bioanalysis, 2014, 6, 2275–2284 CrossRef CAS PubMed.
- Y. Moulard, L. Baily-Chouriberry, S. Boyer, P. Garcia, M. A. Popot and Y. Bonnaire, Anal. Chim. Acta, 2011, 700, 126–136 CrossRef CAS PubMed.
- G. W. Hatfield, S. P. Hung and P. Baldi, Mol. Microbiol., 2003, 47, 871–877 CrossRef CAS PubMed.
- S. Mocellin and C. R. Rossi, Adv. Exp. Med. Biol., 2007, 593, 19–30 CrossRef PubMed.
- J. M. Weiss, E. Simon, G. J. Stroomberg, R. de Boer, J. de Boer, S. C. van der Linden, P. E. G. Leonards and M. H. Lamoree, Anal. Bioanal. Chem., 2011, 400, 3141–3149 CrossRef CAS PubMed.
- Thermo Scientific SIEVE Software for Differential Expression Analysis, http://www.planetorbitrap.com/label-free#tab:data-analysis, accessed August 14th 2015.
- The Association of Official Racing Chemists. AORC Guidelines for the Minimum Criteria for Identification by Chromatography and Mass Spectrometry (January 2015), http://www.aorc-online.org/documents/aorc-ms-criteria-jan-2015/, accessed 20 May 2015.
- National Association of Testing Authorities, Guidelines for the validation and verification of quantitative and qualitative test methods, Technical Note–17 October 2013, http://www.nata.com.au/nata/phocadownload/publications/Guidance_information/tech-notes-information-papers/technical_note_17.pdf, accessed July 4 2014 Search PubMed.
- International Laboratory Accreditation Cooperation, Accreditation requirements and operating criteria for horseracing laboratories. ILAC-G7:06/2009, http://ilac.org/publications-and-resources/ilac-documents/guidance-series/, accessed July 4 2014 Search PubMed.
- R. G. Sadygov, F. M. Maroto and A. F. Hühmer, Anal. Chem., 2006, 78, 8207–8217 CrossRef CAS PubMed.
- M. Askenazi, Recursive base peak framing of mass spectrometry data. United States Patent Application 20060293861, USPTO, http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1%26Sect2=HITOFF%26d=PG01%26p=1%26u=/netahtml/PTO/srchnum.html%26r=1%26f=G%26l=50%26s1=20060293861.PGNR, accessed August 14 2015 Search PubMed.
- M. T. Sheldon, R. Mistrik and T. R. Croley, J. Am. Soc. Mass Spectrom., 2009, 20, 370–376 CrossRef CAS PubMed.
- A. G. Lipomed, http://www.lipomed.com/media/archive1/download/2015-01-13_Flyer-NBOMe-Derivatives.pdf, accessed July 28 2015.
- PiHKAL, http://www.isomerdesign.com/PiHKAL/explore.php?domain=pk%26id=2425, accessed July 28 2015.
- Thermo Fisher Scientific, http://www.mzcloud.org/, accessed September 22 2015.
| This journal is © The Royal Society of Chemistry 2016 |