Electrochemical behavior of eriocitrin and highly sensitive determination based on an electrochemically reduced graphene oxide modified glassy carbon electrode†
Abstract
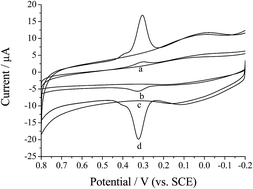
The electrochemical behavior of eriocitrin on the surface of electrochemically reduced graphene oxide (ERGO) was investigated in detail. Based on the significantly enhanced anodic peak current and negatively shifted anodic peak potential of the ERGO modified glassy carbon electrode (ERGO/GCE) compared to that of a bare GCE, ERGO performed as a highly effective catalyst for the electrochemical oxidation of eriocitrin. An electroanalytical method for the determination of eriocitrin was established for the first time using the technique of differential pulse voltammetry (DPV). Under the optimal conditions, a good linear relationship between the anodic peak current and eriocitrin concentration was obtained in the range of 10.0 to 3.5 × 102 ng mL−1 with a detection limit of 0.5 ng mL−1 (S/N = 3). The electrochemical sensor exhibited good selectivity, stability, accuracy and precision. Finally, the proposed method was successfully applied to determine the content of eriocitrin in lemon with satisfactory results.

 Please wait while we load your content...
Please wait while we load your content...