Surface plasmon resonance biosensors for simultaneous monitoring of amyloid-beta oligomers and fibrils and screening of select modulators
Xinyao
Yi
ab,
Chengting
Feng
a,
Shengqiang
Hu
a,
Hengfeng
Li
b and
Jianxiu
Wang
*a
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan, P. R. China 410083. E-mail: jxiuwang@csu.edu.cn
bSchool of Materials Science and Engineering, Central South University, Changsha, Hunan, P. R. China 410083
First published on 18th November 2015
Abstract
Oligomeric amyloid-beta (Aβ) peptides are considered as the most toxic species in Alzheimer's disease (AD). Monitoring of the Aβ aggregation profiles is critical for elucidating the oligomer toxicity and may serve as a therapeutic target for AD. By immobilizing the capture antibodies of A11 and OC that are specific to the oligomers and fibrils, respectively, in separate fluidic channels, a novel surface plasmon resonance (SPR) biosensor was designed for monitoring the oligomeric and fibrillar species of Aβ(1–42) simultaneously. The influence of curcumin, Cu2+ and methylene blue on the amount of toxic oligomers and fibrils was evaluated. The half maximal inhibitory concentration (IC50) of curcumin and methylene blue was determined. The formation of Aβ fibrils was also validated by the thioflavin T (ThT) fluorescence assay. The results demonstrate the utility of SPR as an analytical tool for rapid and comprehensive monitoring of Aβ aggregation and screening of Aβ modulators.
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder and one of its hallmarks is the deposition of neuritic senile plaques in the brains of AD patients. The major constituent of the neuritic plaques is an amyloid-β (Aβ) peptide with 39–43 amino acids cleaved from an amyloid precursor protein by β- and γ-secretases.1,2 Typically, the cerebral deposition of Aβ has been indicated as a key process associated with the progression of AD.3,4 The aggregation of Aβ is a complicated process involving the conversion of soluble monomers to oligomers and fibrils.3 Oligomers were thought to be the most toxic species which disrupt synaptic plasticity and cause neuronal injury.5–7 One way to lower the toxicity is to prevent the production of Aβ by inhibiting the enzymatic activity of β- and γ-secretases.8–10 The use of modulators to inhibit the oligomer formation is another way to reduce the toxicity.4 Thus, the study of Aβ aggregation is related to AD therapy and represents a significant area of AD-related research.To delineate the mechanism of AD progression and to screen select aggregation modulators, monitoring of Aβ aggregation profiles is of great importance. Although a variety of structure- or morphology-based techniques, such as solid-state NMR,11 atomic force microscopy (AFM)12 and X-ray diffraction13 have provided insights into the molecular basis of amyloid aggregation, optical dye-binding techniques are the most frequently used ones to monitor the kinetics of the aggregation process in real time and to screen small-molecule aggregation inhibitors.14 In such an assay, the absorption and/or fluorescence properties of congo red and thioflavin T (ThT) were examined when bound to the aggregates of the amyloidogenic peptides. However, the binding of dyes prevented natural oligomer formation, and the assay is prone to false positive results.15–17 Enzyme-linked immunosorbent assay is another frequently used technique for the detection of Aβ monomers or oligomers. However, it usually suffers from cross-reactivity, nonspecific binding, and low stability of the enzyme conjugates.18,19 Fluorescence resonance energy transfer using a combination of extrinsic fluorophores has also been demonstrated for the detection of amyloid aggregation.20–22 For example, in situ and simultaneous monitoring of the oligomers and fibrils of amyloidogenic peptides was conducted.20 As donors and acceptors, the fluorophores were covalently attached to or mechanically mixed with the amyloidogenic peptides.20–22 However, the feasibility of the assays depends on the distance between the binding sites and the covalent modification of the peptides with the fluorophores making these assays complicated and time-consuming.
Surface plasmon resonance (SPR) serves as a viable alternative for studying the biomolecular interactions and their kinetics.23,24 In contrast to other techniques, SPR is capable of determining the target biomolecules in a real-time and label-free manner.25–27 The aggregation of the Aβ(1–42) peptide from fresh monomers to fully-grown fibrils was analyzed by SPR and AFM.28 The presence of Fe3+ was demonstrated to induce significantly denser aggregates. Compared to the dye-binding assays, SPR does not involve additives or a further labeling step, and a direct assay of the fibrillation of Aβ(1–42) from the initial monomeric species was performed.28 However, the specific forms of Aβ oligomers and fibrils during the aggregation process were not determined by this method. Monitoring of Aβ fibril elongation under different immobilized “seed” conditions was demonstrated by surface plasmon resonance imaging.29 The effect of epigallocatechin-3-gallate and various metal ions (Fe3+, Cu2+, and Zn2+) on the growth of Aβ fibrils was examined. Although multiple assays were achieved, the use of a CCD camera compromises the detection sensitivity.26,30
Veloso et al. studied the aggregation of Aβ by electrochemical impedance spectroscopy using the antibodies of A11 and OC that are specific to the oligomers and fibrils, respectively.31 The surface binding reaction was measured by determining the charge transfer resistance of the [Fe(CN)6]3−/4− redox probe. The sym-triazine-derived aggregation modulators to reduce the amount of toxic oligomers were examined. However, in that work, the sensing protocol is complicated and the results are uncomparable because the A11 and OC antibodies were modified onto different electrodes and the antigen–antibody interaction was not measured simultaneously.
In this study, a SPR biosensor for analyzing Aβ aggregation was designed. Oligomeric and fibrillar species of Aβ(1–42) were captured by their respective antibodies immobilized onto different fluidic channels of one chip and the aggregation profiles were attained. The effect of several modulators on the distribution of the oligomeric and fibrillar species was also examined. The sensing protocol enables the monitoring of the Aβ aggregation process on two channels of one single chip, and consequently the assay accuracy and reproducibility were significantly improved.
Experimental section
Chemicals and materials
16-Mercaptohexadecan-1-ol was purchased from Frontier Scientific, Inc. (Logan, Utah). Diethylene glycol dimethyl ether, epichlorohydrin, dextran, bromoacetic acid, N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), ethanolamine hydrochloride (EA), NaH2PO4, and Na2HPO4 were acquired from Sigma (St. Louis, MO). A11 antibody that is specific to the oligomers and OC antibody which recognizes the fibrils were obtained from Millipore Inc. (Dedham, MA). Aβ(1–42) was purchased from American Peptide Inc. (Sunnyvale, CA). Curcumin, methylene blue, and CuCl2 were obtained from Sinopharm Chemical Reagent Co., Ltd (China). Other reagents were of analytical purity and used as received. All stock solutions were prepared daily with deionized water treated with a water purification system (Simplicity 185, Millipore Corp., Billerica, MA). The stock solutions of Aβ(1–42) (0.2 mM) and the antibodies were diluted with the phosphate buffer (10 mM phosphate, pH 7.4). The diluted Aβ(1–42) solution was then incubated alone and with curcumin or Cu2+ or methylene blue for different time periods.Instruments
The SPR measurements were conducted on a BI-SPR 3000 system (Biosensing Instrument Inc., Tempe, AZ). The phosphate buffer was degassed under vacuum for 30 min and used as the carrier solution. The fluorescence emission spectra were obtained on a Hitachi F-4600 spectrofluorometer (Hitachi, Japan).Procedures
Au films coated onto BK7 glass slides were purchased from Biosensing Instrument Inc. (Tempe, AZ) and annealed in a hydrogen flame to eliminate surface contaminants. The carboxymethylated dextran (CM-dextran)-covered SPR chips were prepared according to the literature reported.32Via the amide bond formation, channel 1 and channel 2 were coated with A11 and OC antibodies, respectively. Both the channels were treated with 1 M EA to block the empty sites. The incubated samples of Aβ(1–42) in the absence and presence of curcumin or Cu2+ or methylene blue were preloaded into a 200 μL sample loop on an injection valve and then delivered to the flow cell by a syringe pump (Model KDS260, KD Scientific, Holliston, MA). The flow rate was maintained at 20 μL min−1.Results and discussion
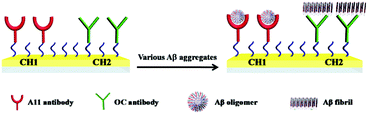
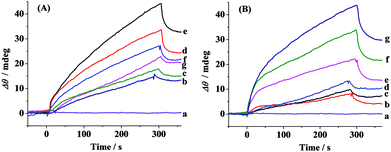
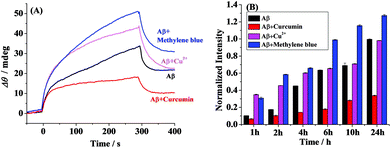
The schematic diagram of the simultaneous SPR determination of Aβ(1–42) oligomers and fibrils is illustrated in Fig. 1. Modifying the Au chips with CM-dextran molecules reduces the nonspecific adsorption of the various forms of Aβ. The A11 and OC antibodies were individually immobilized onto the CM-dextran-covered chips via amine coupling. The A11 antibody recognizes the epitopes of the toxic oligomeric intermediates of the amyloidogenic proteins, and does not recognize the monomers, dimers, trimers, tetramers and fibrils.33 Meanwhile the OC antibody reacts specifically with the fibrils.34 When the various Aβ aggregates or the mixture of Aβ aggregates with select modulators were flowed over the sensor surface, the oligomers and fibrils were captured by their corresponding antibodies which were immobilized onto separate fluidic channels. The molecular weight of the oligomers or fibrils is high enough to produce a reasonable SPR signal. The sensor chip can be regenerated with NaOH for multiple and continuous SPR assays on one chip. Thus, SPR provides a rapid and comprehensive means to monitor the various forms of Aβ, to evaluate the pathways of Aβ assembly and to screen the candidate compounds that modulate the aggregation process of Aβ.The aggregation of Aβ(1–42) was stimulated by incubation under physiological conditions at various time points. At each measured time point, the samples were serially flowed over the two channels to measure the distribution of the oligomers and fibrils. Time-dependent SPR sensorgrams upon injection of the incubated samples were acquired at the sensor chips pre-immobilized with A11 antibody (Fig. 2A) and OC antibody (Fig. 2B). The difference in the baseline SPR angles before and after the injection reflects the amount of the bound species. The nonspecific adsorption (2–3 mdeg) was subtracted via the injection of the incubated samples onto the CM-dextran-covered SPR chip. At the time point of 0 h, no SPR signals in curves a of Fig. 2A and B were expected because the incubated samples are monomer-dominated, and the monomers cannot be recognized by the A11 or OC antibody. With the increase of the incubation time, a gradual increase in the SPR signals in Fig. 2B was obtained, indicating an increased tendency of fibril formation. The highest SPR signal was obtained at 24 h, and beyond 24 h, the SPR signals remain unchanged, indicating a saturated aggregation process. Such a trend is in accordance with the predicted nucleation-dependent process of Aβ aggregation.35 As for CH1, the SPR signals at the A11 antibody-covered surface increased with the incubation time less than 6 h (curves a–e, Fig. 2A), suggesting that the oligomers were gradually formed with time. However, when the incubation time exceeds 6 h, the SPR signals decreased (curves f and g, Fig. 2A). This was caused by further aggregation of the oligomers to amorphous forms, protofibrils or fibrils, which cannot be recognized by the A11 antibody. Monitoring of Aβ aggregation profiles on one single chip leads to excellent reproducibility and accuracy of the assay. SPR thus provides a viable alternative for monitoring of the aggregation process of Aβ(1–42).
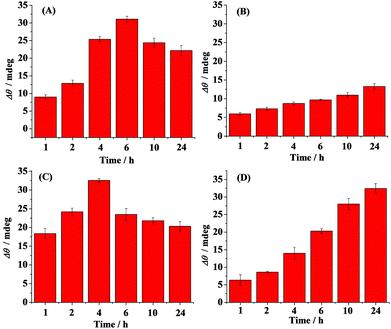
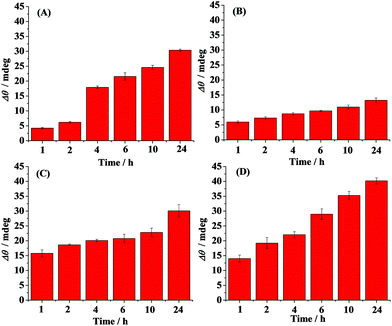
Time-dependent distribution of the oligomers in the absence (A) and presence of curcumin (B), Cu2+ (C) and methylene blue (D) is shown in Fig. 3. Curcumin, Cu2+ and methylene blue were chosen as modulators of Aβ aggregation in this study. The amount of the oligomers increased with the incubation time and reached the maximum value at 6 h (Fig. 3A). Beyond 6 h, the oligmers were gradually aggregated to fibrils, as evidenced by the decreased SPR signals at the A11 antibody-covered surface (CH1) (Fig. 2A and 3A). Curcumin was used as a classic modulator which suppresses the morbidity of AD.36,37 The treatment of Aβ(1–42) with curcumin results in a much smaller SPR signal (Fig. 3B), indicating that curcumin is a potential inhibitor of Aβ(1–42) oligomer formation. Cu2+ is capable of binding with Aβ, which accelerates the aggregation process of Aβ.38,39 As shown in Fig. 3C, Cu2+-treated Aβ(1–42) appeared to promote the oligomer formation, because the maximum SPR signal was achieved after 4 h incubation. The above results are in agreement with the previous reports that curcumin suppressed the oligomer formation and Cu2+ improved the oligomerization of Aβ.29,36,40 As a leading compound in drug research, methylene blue has attracted much attention for its ability to modulate amyloid fibrillation.41 When adding methylene blue into the Aβ(1–42) solution, we found that the oligomer formation was increased slowly and the maximum SPR signal was obtained after incubation for 24 h (Fig. 3D). Thus, methylene blue lowered the aggregation rate of Aβ(1–42) from monomers to oligomers or promoted the aggregation rate of oligomers to fibrils, which decreased the amount of toxic oligomers.
The influence of curcumin, Cu2+ and methylene blue on the fibril formation was also studied. Fig. 4 shows the time-dependent distribution of the fibrils in the absence (A) and presence of curcumin (B), Cu2+ (C) and methylene blue (D). A gradual increase in the SPR signals was observed upon injection of the Aβ(1–42) solution onto the fibril-specific OC antibody-covered SPR chip, and a maximum value was obtained after incubation for 24 h (Fig. 4A and 2B). In the presence of curcumin, the tendency for fibril formation was much lower (Fig. 4B), suggesting that curcumin inhibits the formation of fibrils. Such an inhibition effect may be ascribed to the formation of a lower amount of oligomers (Fig. 3B). In contrast, the presence of Cu2+ accelerated the fibril formation within 2 h, and beyond 2 h, the tendency began to level off (Fig. 4C). In comparison with that in the case of Aβ(1–42) alone, methylene blue promoted the fibril formation and a gradual increase in the SPR signals was attained (Fig. 4D).
Taken together, curcumin suppressed the formation of both oligomers and fibrils, while Cu2+ served as a promoter of Aβ aggregation within a shorter incubation time period (∼2 h). For methylene blue, it promoted the aggregation of Aβ(1–42) oligomers to fibrils. The above results are in good agreement with those reported.36–39,42 For example, Cole and coworkers and Palumaa and coworkers demonstrated that curcumin was an oligomer-specific inhibitor and hindered the fibril formation.36,37 Cu2+ could dramatically induce Aβ aggregation and treatment with a Cu2+ chelator markedly and rapidly inhibiting the process.38,39 Methylene blue was shown as a promoter of Aβ aggregation by Glabe and coworkers, which inhibited Aβ oligomerization by promoting the fibrillization process.42 Thus, the oligomerization and fibrillization pathways of Aβ(1–42) are distinct and methylene blue stabilizes the fibrillized conformation.42
For clarity, we compared the SPR responses at the OC antibody-covered SPR chips (CH2) upon incubation of Aβ(1–42) in the absence and presence of curcumin or Cu2+ or methylene blue for 6 h (Fig. 5A). The highest SPR signal was obtained in the presence of methylene blue, again indicating that methylene blue is a promoter of the fibril formation. However, the presence of the inhibitor of curcumin results in a much smaller SPR signal. Cu2+ serves as a promoter of the fibril formation within 2 h (Fig. 4C), and similar SPR responses were observed in the absence and presence of Cu2+ upon incubation for 6 h (Fig. 5A). To further validate the above data, the ThT fluorescence assay was performed (Fig. 5B). ThT, which has been widely used for monitoring of Aβ aggregation, associates rapidly with the aggregated fibrils.35 The comparative analysis indicates that the overall trend by the ThT assay matched closely with that by SPR at numerous time points, demonstrating the validity of the proposed method. It is worth noting that the accuracy for the SPR assay of both oligomers and fibrils on one single chip was significantly improved. Furthermore, excellent reproducibility and throughput could be achieved via chip regenerations. Therefore, SPR may serve as a reasonable means for monitoring the formation and distribution of the various Aβ aggregates.
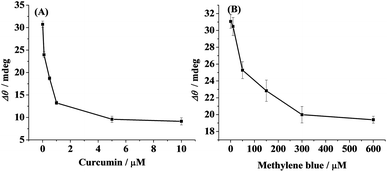
Finally, the inhibition assay of curcumin (A) and methylene blue (B) on the oligomer formation was conducted (Fig. 6). The SPR signals were remarkably decreased at lower concentrations and then plateaued at the elevated ones. IC50, the half maximal inhibitory concentration, was estimated to be 0.7 μM for curcumin and 37 μM for methylene blue. Kim et al. also reported that curcumin had a strong inhibitory effect on Aβ oligomer formation (IC50 = 0.68 μM) by performing the ThT assay.43 Thus, SPR is capable of monitoring of Aβ aggregation and screening of select inhibitors.
Conclusions
A SPR biosensor for simultaneously monitoring the distribution of Aβ(1–42) oligomers and fibrils in one single assay has been developed. The employment of the A11 and OC antibodies, which recognize the conformations of the oligomers and fibrils, respectively, enables the multiplexed capability of the assay. The proposed method has been utilized for efficient screening of select inhibitors and promoters. The presence of curcumin remarkably disrupted the aggregation process of Aβ(1–42), resulting in a decrease in the amount of both oligomers and fibrils. In contrast, Cu2+ was found to increase the formation of both oligomers and fibrils within a shorter incubation time period (∼2 h), while methylene blue promoted the fibrillization of Aβ by inhibiting the oligomerization process. Though only the oligomers and fibrils were determined, we envision that the multiplexed assay of various species, such as monomers, oligomers, protofibrils, and fibrils can be realized by multiple SPR channels. Thus, SPR serves as an effective means for monitoring Aβ aggregation profiles and high-throughput screening of inhibitors and promoters.Acknowledgements
The authors thank the National Natural Science Foundation of China (no. 21175156, 21575166 and 21375150) and China Postdoctoral Science Foundation (no. 2015M580696) for support. We also thank Dr Feimeng Zhou at California State University, Los Angeles for his useful comments.Notes and references
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS PubMed.
- D. J. Selkoe, Behav. Brain Res., 2008, 192, 106–113 CrossRef CAS PubMed.
- C. Haass, M. G. Schlossmacher, A. Y. Hung, C. Vigopelfrey, A. Mellon, B. L. Ostaszewski, I. Lieberburg, E. H. Koo, D. Schenk, D. B. Teplow and D. J. Selkoe, Nature, 1992, 359, 322–325 CrossRef CAS PubMed.
- D. M. Walsh, I. Klyubin, J. V. Fadeeva, W. K. Cullen, R. Anwyl, M. S. Wolfe, M. J. Rowan and D. J. Selkoe, Nature, 2002, 416, 535–539 CrossRef CAS PubMed.
- Y. Gong, L. Chang, K. L. Viola, P. N. Lacor, M. P. Lambert, C. E. Finch, G. A. Krafft and W. L. Klein, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10417–10422 CrossRef CAS PubMed.
- C. A. McLean, R. A. Cherny, F. W. Fraser, S. J. Fuller, M. J. Smith, K. Beyreuther, A. I. Bush and C. L. Masters, Ann. Neurol., 1999, 46, 860–866 CrossRef CAS PubMed.
- L. F. Lue, Y. M. Kuo, A. E. Roher, L. Brachova, Y. Shen, L. Sue, T. Beach, J. H. Kurth, R. E. Rydel and J. Rogers, Am. J. Pathol., 1999, 155, 853–862 CrossRef CAS PubMed.
- J. E. Ladbury, Biochem. Soc. Trans., 2010, 38, 888–893 CrossRef CAS PubMed.
- S. Sinha, J. P. Anderson, R. Barbour, G. S. Basi, R. Caccavello, D. Davis, M. Doan, H. F. Dovey, N. Frigon, J. Hong, K. Jacobson-Croak, N. Jewett, P. Keim, J. Knops, I. Lieberburg, M. Power, H. Tan, G. Tatsuno, J. Tung, D. Schenk, P. Seubert, S. M. Suomensaari, S. W. Wang, D. Walker, J. Zhao, L. McConlogue and V. John, Nature, 1999, 402, 537–540 CrossRef CAS PubMed.
- R. Vassar, Adv. Drug Delivery Rev., 2002, 54, 1589–1602 CrossRef CAS PubMed.
- K. Iwata, T. Fujiwara, Y. Matsuki, H. Akutsu, S. Takahashi, H. Naiki and Y. Goto, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 18119–18124 CrossRef CAS PubMed.
- I. A. Mastrangelo, M. Ahmed, T. Sato, W. Liu, C. P. Wang, P. Hough and S. O. Smith, J. Mol. Biol., 2006, 358, 106–119 CrossRef CAS PubMed.
- M. Sunde, L. C. Serpell, M. Bartlam, P. E. Fraser, M. B. Pepys and C. C. Blake, J. Mol. Biol., 1997, 273, 729–739 CrossRef CAS PubMed.
- L. P. Jameson, N. W. Smith and S. V. Dzyuba, ACS Chem. Neurosci., 2012, 3, 807–819 CrossRef CAS PubMed.
- M. Groenning, J. Chem. Biol., 2010, 3, 1–18 CrossRef PubMed.
- F. Meng, P. Marek, K. J. Potter, C. B. Verchere and D. P. Raleigh, Biochemistry, 2008, 47, 6016–6024 CrossRef CAS PubMed.
- B. Y. Feng, B. H. Toyama, H. Wille, D. W. Colby, S. R. Collins, B. C. H. May, S. B. Prusiner, J. Weissman and B. K. Shoichet, Nat. Chem. Biol., 2008, 4, 197–199 CrossRef CAS PubMed.
- H. Hillen, S. Barghorn, A. Striebinger, B. Labkovsky, R. Muller, V. Nimmrich, M. W. Nolte, C. Perez-Cruz, I. van der Auwera, F. van Leuven, M. van Gaalen, A. Y. Bespalov, H. Schoemaker, J. P. Sullivan and U. Ebert, J. Neurosci., 2010, 30, 10369–10379 CrossRef CAS PubMed.
- A. C. Klaver, L. M. Patrias, M. P. Coffey, J. M. Finke and D. A. Loeffler, J. Neurosci. Methods, 2010, 187, 263–269 CrossRef CAS PubMed.
- B. Alies, H. Eury, M. Essassi el, G. Pratviel, C. Hureau and P. Faller, Anal. Chem., 2014, 86, 11877–11882 CrossRef CAS PubMed.
- J. Lee, E. K. Culyba, E. T. Powers and J. W. Kelly, Nat. Chem. Biol., 2011, 7, 602–609 CrossRef CAS PubMed.
- S. D. Quinn, P. A. Dalgarno, R. T. Cameron, G. J. Hedley, C. Hacker, J. M. Lucocq, G. S. Baillie, I. D. W. Samuel and J. C. Penedo, Mol. BioSyst., 2014, 10, 34–44 RSC.
- N. Xia, L. Liu, M. G. Harrington, J. Wang and F. Zhou, Anal. Chem., 2010, 82, 10151–10157 CrossRef CAS PubMed.
- X. Yi, Y. Hao, N. Xia, J. Wang, M. Quintero, D. Li and F. Zhou, Anal. Chem., 2013, 85, 3660–3666 CrossRef CAS PubMed.
- J. Homola, Chem. Rev., 2008, 108, 462–493 CrossRef CAS PubMed.
- M. J. Linman, A. Abbas and Q. A. Cheng, Analyst, 2010, 135, 2759–2767 RSC.
- S. Wang, S. Boussaad and N. J. Tao, Rev. Sci. Instrum., 2001, 72, 3055–3060 CrossRef CAS.
- J. Ryu, H.-A. Joung, M.-G. Kim and C. B. Park, Anal. Chem., 2008, 80, 2400–2407 CrossRef CAS PubMed.
- X. R. Cheng, B. Y. Hau, A. J. Veloso, S. Martic, H. B. Kraatz and K. Kerman, Anal. Chem., 2013, 85, 2049–2055 CrossRef CAS PubMed.
- G. D. VanWiggeren, M. A. Bynum, J. P. Ertel, S. Jefferson, K. A. Robotti, E. P. Thrush, D. A. Baney and K. P. Killeen, Sens. Actuator, B, 2007, 127, 341–349 CrossRef CAS.
- A. J. Veloso, A. M. Chow, H. V. Ganesh, N. Li, D. Dhar, D. C. Wu, S. Mikhaylichenko, I. R. Brown and K. Kerman, Anal. Chem., 2014, 86, 4901–4909 CrossRef CAS PubMed.
- S. Lofas, Pure Appl. Chem., 1995, 67, 829–834 CrossRef CAS.
- R. Kayed, E. Head, J. L. Thompson, T. M. McIntire, S. C. Milton, C. W. Cotman and C. G. Glabe, Science, 2003, 300, 486–489 CrossRef CAS PubMed.
- R. Kayed, E. Head, F. Sarsoza, T. Saing, C. W. Cotman, M. Necula, L. Margol, J. Wu, L. Breydo, J. L. Thompson, S. Rasool, T. Gurlo, P. Butler and C. G. Glabe, Mol. Neurodegener., 2007, 2, 18 CrossRef PubMed.
- S. W. Snyder, U. S. Ladror, W. S. Wade, G. T. Wang, L. W. Barrett, E. D. Matayoshi, H. J. Huffaker, G. A. Krafft and T. F. Holzman, Biophys. J., 1994, 67, 1216–1228 CrossRef CAS PubMed.
- F. Yang, G. P. Lim, A. N. Begum, O. J. Ubeda, M. R. Simmons, S. S. Ambegaokar, P. P. Chen, R. Kayed, C. G. Glabe, S. A. Frautschy and G. M. Cole, J. Biol. Chem., 2005, 280, 5892–5901 CrossRef CAS PubMed.
- K. Zovo, E. Helk, A. Karafin, V. Tougu and P. Palumaa, Anal. Chem., 2010, 82, 8558–8565 CrossRef CAS PubMed.
- C. S. Atwood, R. D. Moir, X. Huang, R. C. Scarpa, N. M. Bacarra, D. M. Romano, M. A. Hartshorn, R. E. Tanzi and A. I. Bush, J. Biol. Chem., 1998, 273, 12817–12826 CrossRef CAS PubMed.
- R. A. Cherny, C. S. Atwood, M. E. Xilinas, D. N. Gray, W. D. Jones, C. A. McLean, K. J. Barnham, I. Volitakis, F. W. Fraser, Y. S. Kim, X. D. Huang, L. E. Goldstein, R. D. Moir, J. T. Lim, K. Beyreuther, H. Zheng, R. E. Tanzi, C. L. Masters and A. I. Bush, Neuron, 2001, 30, 665–676 CrossRef CAS PubMed.
- X. Huang, M. P. Cuajungco, C. S. Atwood, M. A. Hartshorn, J. D. A. Tyndall, G. R. Hanson, K. C. Stokes, M. Leopold, G. Multhaup, L. E. Goldstein, R. C. Scarpa, A. J. Saunders, J. Lim, R. D. Moir, C. Glabe, E. F. Bowden, C. L. Masters, D. P. Fairlie, R. E. Tanzi and A. I. Bush, J. Biol. Chem., 1999, 274, 37111–37116 CrossRef CAS PubMed.
- M. Oz, D. E. Lorke and G. A. Petroianu, Biochem. Pharmacol., 2009, 78, 927–932 CrossRef CAS PubMed.
- M. Necula, L. Breydo, S. Milton, R. Kayed, W. E. van der Veer, P. Tone and C. G. Glabe, Biochemistry, 2007, 46, 8850–8860 CrossRef CAS PubMed.
- H. Kim, B. S. Park, K. G. Lee, C. Y. Choi, S. S. Jang, Y. H. Kim and S. E. Lee, J. Agric. Food Chem., 2005, 53, 8537–8541 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |