Deterioration mechanism of electrochromic poly(3,4-(2,2-dimethylpropylenedioxy)thiophene) thin films†
Abstract
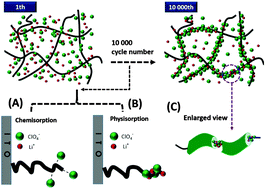
A long lifetime of electrochromic (EC) materials is required in real-life applications, and the understanding and alleviation of deterioration are imperative to their commercialization. Here we investigate the stability of poly(3,4-(2,2-dimethylpropylenedioxy)thiophene) (PProDot-Me2) films via cycling 10 000 times in 0.1 M LiClO4/propylene carbonate (PC). Scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy results reveal that parts of [ClO4]− and Li+ ions were trapped and then adsorbed on polymer fibres, which makes the morphology change from irregular networks to compact microstructures, and the inclusion complex formed reduces the number of electroactive sites and blocks ions channels, preventing the migration of free [ClO4]− from the electrolyte. Based on our hypothesis, the stability was finally improved by optimizing the cycling conditions. This study provides guidance to improve the stability of EC materials.

 Please wait while we load your content...
Please wait while we load your content...