A superior low-cost amorphous carbon anode made from pitch and lignin for sodium-ion batteries†
Yunming
Li
,
Yong-Sheng
Hu
*,
Hong
Li
,
Liquan
Chen
and
Xuejie
Huang
Key Laboratory for Renewable Energy, Beijing Key Laboratory for New Energy Materials and Devices, Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yshu@aphy.iphy.ac.cn
First published on 9th November 2015
Abstract
Sodium-ion batteries (SIBs) are a promising candidate for grid electricity storage due to their potential low cost. The development of anode materials is a crucial step to promote the commercialization of SIBs, and amorphous carbon materials are likely to be the most promising alternatives among all proposed anode materials. However, the cost of the reported carbon materials is still very high due to the expensive precursors and their low carbon yield. Here, we report an amorphous carbon (AC) material made from low cost pitch. The amorphous carbon material with an amazing high carbon yield of 57% was achieved by utilizing the emulsification interaction between pitch and lignin to suppress the graphitization of pitch during the carbonization. The effects of heat-treatment temperatures and the pitch/lignin mass ratios on the morphology, microstructure and the electrochemical performance of AC were systematically investigated. By optimizing experimental conditions, we achieved one representative AC with a suitable morphology and microstructure, which exhibits promising performances with a high reversible capacity of 254 mA h g−1, a high initial coulombic efficiency of 82% and excellent cycling stability. This is the first demonstration that the pitch can be successfully applied in fabricating amorphous carbon anode materials for SIBs with superior low cost and high performance.
Introduction
Recently, with the gradual deterioration of the ecological environment, the efficient utilization of renewable energy resources has already become an urgent task for creating a healthy eco-environment and a sustainable energy structure. The development of low-cost and high-performance energy storage systems (EESs) is indispensable for guaranteeing a stable and continuous energy supply. Among secondary batteries, lithium-ion batteries (LIBs), which have been successfully developed as power sources for portable electronic devices, are a promising alternative source due to their highest energy/power density and long cycle life.1 However, the rarity and non-uniform geographic distribution of lithium resources may severely restrict LIBs' prospective applications in grid-scale energy storage.2,3 Sodium-ion batteries (SIBs) have recently been considered as an alternative energy storage technology to LIBs due to their potentially low cost and the widespread reserves of sodium resources.4–8 Furthermore, Na+ ions can be used as the charge carrier to explore new redox reactions and new materials to further decrease the cost. For instance, aluminum foil can be used as the anode current collector because sodium cannot form the alloy with aluminum, leading to no performance decay during overdischarging.Until now, a number of cathode materials have been proposed, making the production of high-performance SIBs possible. They mainly include layered oxides (P2 and O3),9–13 tunnel-type oxides,14,15 phosphates16,17 and sulphates.18 Recently, Hu et al. reported air-stable copper-based P2–Na7/9Cu2/9Fe1/9Mn2/3O2 and O3–Na0.9[Cu0.22Fe0.30Mn0.48]O2, which show very promising application in rechargeable batteries with only environmentally friendly and low-cost elements.19,20
With respect to anodes, graphite which has been widely used as an anode material for LIBs shows poor Na storage performance due to the unique feature of the large and highly ionized Na+ ion.21 The widely studied anodes for SIBs mainly include carbonaceous materials, alloys,22 Ti-based oxides and organic compounds. Na alloy anode materials of Sb/C,23 Sn/C (ref. 24) and SnSb/C (ref. 25) exhibit a high capacity of 610 mA h g−1, 295 mA h g−1 and 540 mA h g−1, respectively. However, the structure destruction caused by the large volume expansion in the reaction with Na limits their cycling stability.26 Ti-based oxides mainly include Na2Ti3O7,27 Li4Ti5O12 (ref. 28 and 29) and Na0.66[Li0.22Ti0.78]O2,30 but either the storage capacity or low coulombic efficiency hinders their applications. Na2C8H4O4 is a typical organic compound studied as the anode for SIBs, and it delivers a large reversible capacity with good capacity retention.31 Nevertheless, insufficient electronic conductivity and low initial coulombic efficiency limit the further development of organic compounds. Among anode candidates for SIBs, carbonaceous materials hold the most promise considering the resource and cost. Recently, graphite has been studied as a suitable anode for SIBs, the reversible Na insertion and extraction was realized by either enlarging the interlayer distance of graphite or Na+-solvent co-intercalation, but the first coulombic efficiency is very low.32–34 Hard carbon, also known as non-graphitizable carbon, shows promising performances with low operating potential and relatively high specific capacity as the anode for SIBs because of its disordered structure with a large interlayer distance.35–39 Unfortunately, the low initial coulombic efficiency and the high cost limit its application. The poor initial coulombic efficiency caused by the formation of a solid electrolyte interphase (SEI) layer which results in the irreversible loss of Na provided by the cathode would ultimately lead to capacity decrease in the full cell. Hard carbon is usually prepared through pyrolysis of different carbonaceous precursors, such as polymers, sugars, and so on. However, their relatively high cost and low carbon yield (Table 1) give rise to the high cost of hard carbon. Therefore, how to improve the carbon yield and reduce the cost has become a key step to satisfy the requirement for practical applications.
| Precursor | Pitch | Sucrose | Lignin | Phenolic resin | Starch | Cellulose |
|---|---|---|---|---|---|---|
| a http://www.alibaba.com/. | ||||||
| Price ($ per ton) | 300 | 400 | 450 | 2000 | 500 | 1000 |
| Carbon yield (%) | 56 | <10 | 43 | 47 | <10 | <10 |
As a low-cost petrochemical by-product, pitch is widely used as the carbon source for soft carbon and artificial graphite because of the low cost and high carbon content. In 2011, Adelhelm et al. first reported a pitch-derived carbon as the anode for SIBs, but it only delivers a storage capacity of about 130 mA h g−1, and the initial coulombic efficiency is only 14%.40 It would be an important breakthrough for the development of low-cost SIBs if we can succeed in fabricating carbonaceous anode materials with high performance using pitch as the precursor. Our very recent work reported an amorphous carbon anode from pitch and phenolic resin, but phenolic resin is expensive (Table 1).41 Lignin, the second most abundant organic material in nature, is a very suitable hard carbon precursor with the features of low cost and abundance. Furthermore, lignin is water soluble and can emulsify pitch, which means that they can crosslink with each other. Herein, we aim to combine the advantages from both soft carbon precursors and hard carbon precursors to produce an amorphous carbon (AC) material with low cost, high carbon yield and high performance by using the emulsification interaction between pitch and lignin to suppress the graphitization of pitch in the process of high temperature carbonization. Ultimately, an amorphous carbon material was successfully obtained through pyrolysis of such a mixture of pitch and lignin. When evaluated as an anode for SIBs, the pitch/lignin derived carbon exhibits a high coulombic efficiency, a relatively high storage capacity and stable cycling performance. It turns out that we introduced an effective strategy to use pitch to prepare amorphous carbon with superior low cost and excellent electrochemical performance, and the method is also suitable for industrial production for its simplicity and high carbon yield.
Experimental
Materials synthesis
AC was prepared by pyrolysis of the mixture of pitch and lignin. The precursor materials of pitch and lignin (Ourchem Co. China, 96%) with different proportions were mixed in aqueous solutions by the ball-milling dispersion method and then the slurry was dried at 100 °C for 24 h to remove the water. The obtained black powder was carbonized for 2 hours in a tube furnace under argon flow. The carbonization temperatures were 1200 °C, 1400 °C and 1600 °C, respectively. The fabricated amorphous carbon materials denoted as AC111200, AC111400, AC111600, AC731400 and AC371400 were prepared as summarized in Table 2.| Sample | AC111200 | AC111400 | AC111600 | AC731400 | AC371400 | Pitch |
|---|---|---|---|---|---|---|
| a The heat treatment temperatures. b The initial charge capacity. c The capacity percentage of the plateau region. d The initial coulombic efficiency. | ||||||
| Pitch/lignin | 1/1 | 1/1 | 1/1 | 7/3 | 3/7 | 0 |
| HTTsa (°C) | 1200 | 1400 | 1600 | 1400 | 1400 | 1400 |
| d 002 (Å) | 3.84 | 3.61 | 3.52 | 3.57 | 3.66 | 3.45 |
| L c (nm) | 1.64 | 2.14 | 2.86 | 2.52 | 1.92 | 7.39 |
| L a (nm) | 4.13 | 4.00 | 3.78 | 4.09 | 3.73 | 5.53 |
| S BET (m2 g−1) | 4.29 | 1.34 | 4.55 | 1.74 | 34.74 | 2.85 |
| ICCb (mA h g−1) | 247 | 254 | 205 | 200 | 246 | 85 |
| CPPc (%) | 60 | 65 | 71 | 62 | 70 | 0 |
| ICEd (%) | 82 | 82 | 75 | 78 | 74 | 58 |
Materials characterization
The structure was characterized by using an X'Pert Pro MPD X-ray diffractometer (XRD) (Philips, Netherlands) using Cu-Kα radiation (1.5405 Å) and Raman spectra (JY-HR 800). The morphologies of the samples were investigated with a scanning electron microscope (SEM) (Hitachi S-4800). High-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) patterns were recorded on a FEI Tecnai F20 transmission electron microscope. TGA data were obtained using a NETZSCH STA 409 PC Luxx simultaneous thermal analyser (Germany) from room temperature to 1000 °C at a heating rate of 5 °C min−1 under a nitrogen gas atmosphere. Nitrogen adsorption and desorption isotherms were determined by nitrogen physisorption on a Micromeritics ASAP 2020 analyzer.Electrochemical measurements
All the electrochemical tests were conducted in coin cells (CR2032). The working electrode was prepared by spreading the mixed slurry of the active material and sodium alginate binder in water solvent with a weight ratio of 9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 onto Al foil, and then dried at 100 °C in a vacuum for 10 hours. The electrolyte was a solution of 0.6 M NaPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1
0.5 onto Al foil, and then dried at 100 °C in a vacuum for 10 hours. The electrolyte was a solution of 0.6 M NaPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume). A sodium foil was used as the counter electrode and glass fiber was used as the separator. All the operations were operated in an argon-filled glovebox. The discharge and charge tests were carried out on a Land BT2000 battery test system (Wuhan, China) in a voltage range of −0.01–2 V at various C-rates at room temperature. A sodium-ion full cell was constructed using the AC111400 sample as the anode and O3–Na0.9[Cu0.22Fe0.30Mn0.48]O2 as the cathode in a CR2032 coin-type cell. The synthesis method of the Na0.9[Cu0.22Fe0.30Mn0.48]O2 material was a conventional solid state reaction.20 The weight ratio of the two electrodes (anode/cathode) was 1
1 in volume). A sodium foil was used as the counter electrode and glass fiber was used as the separator. All the operations were operated in an argon-filled glovebox. The discharge and charge tests were carried out on a Land BT2000 battery test system (Wuhan, China) in a voltage range of −0.01–2 V at various C-rates at room temperature. A sodium-ion full cell was constructed using the AC111400 sample as the anode and O3–Na0.9[Cu0.22Fe0.30Mn0.48]O2 as the cathode in a CR2032 coin-type cell. The synthesis method of the Na0.9[Cu0.22Fe0.30Mn0.48]O2 material was a conventional solid state reaction.20 The weight ratio of the two electrodes (anode/cathode) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.65. The full cells were charged and discharged in a voltage range of 1–4.06 V at 0.2C current rate. The energy density was calculated by dividing the discharge energy value by the total mass of cathode and anode materials.
2.65. The full cells were charged and discharged in a voltage range of 1–4.06 V at 0.2C current rate. The energy density was calculated by dividing the discharge energy value by the total mass of cathode and anode materials.
Results and discussion
It is interesting to find that the lignin can emulsify pitch. We first mixed pitch with lignin in aqueous solutions to make them fully interact with each other before the carbonization process. The mixture solutions containing various pitch/lignin mass ratios of 7/3, 1/1 and 3/7 were prepared considering the disordered degree and carbon yield of amorphous carbon. The microstructure of amorphous carbon may have a remarkable effect on Na storage behavior, ultimately determining the electrochemical performance of carbonaceous anode materials for SIBs. Therefore, we also prepared amorphous carbon materials with different heat-treatment temperatures (HTTs) to investigate the influence of carbonized temperature on the carbon structure ordering and electrochemical performance. Finally, five representative amorphous carbon materials with different degrees of carbon structure ordering were obtained and compared.The morphologies of AC under various conditions are shown in Fig. 1a–e. All pitch/lignin derived carbon materials are found to have a granular morphology with 2–10 μm in size, and non-uniform distribution of the particle size is noted. The particle grows up gradually and becomes uniform and regular with increasing HTTs from 1200 to 1600 °C. With increasing addition of the lignin content, the morphology becomes from flake to granular and the particle size decreases, which indicates that the lignin is beneficial to restrain the growth of AC.
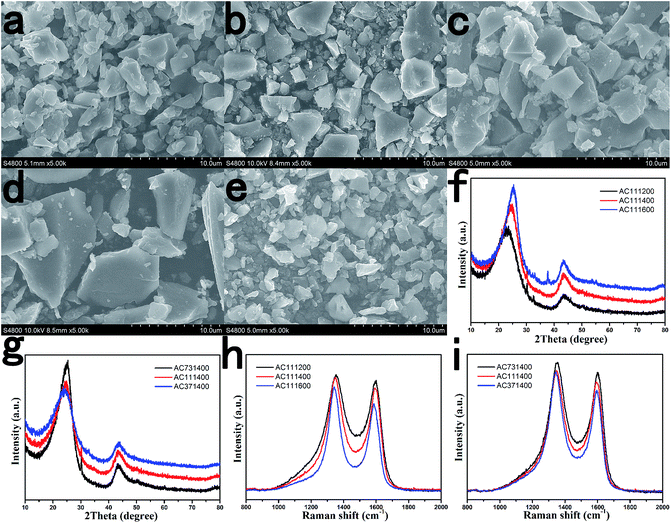 | ||
| Fig. 1 Morphologies of (a) AC111200, (b) AC111400, (c) AC111600, (d) AC731400 and (e) AC371400 observed by SEM; (f and g) XRD data and (h and i) Raman spectra of AC under different conditions. | ||
The microstructure of AC is further characterized by X-ray diffraction (XRD) and Raman spectroscopy, and the results are shown in Fig. 1f–i. All XRD patterns show broad peaks at 24° and 43°, which are attributed to the crystallographic planes of (002) and (100) in the disordered carbon structure. The (002) peak position shifts to a higher angle and the peak becomes narrower with increasing HTTs, indicating that d002 decreases and Lc increases due to the structural development from disordering to short-range ordering at higher HTTs. In addition, the average interplanar spacing d002 increases with decreasing mass ratios of pitch to lignin, suggesting that the lignin addition introduces a large amount of topological defects to the AC. Raman spectra present two separate characteristic bands of the D-band peak at 1343 cm−1 (the defect-induced band) and the G-band peak at 1589 cm−1 (the crystalline graphite band).42 The half width at half maximum (HWHM) of G and D bands in the Raman spectra decreases slightly with increasing HTTs, which indicates the development of an ordered hexagonal structure. Furthermore, the AC carbonized at higher temperature has a smaller La that is calculated from the intensity ratio of D-band to G-band (ID/IG), suggesting that the (100) direction of small graphitic crystals shrinks at a higher temperature during the carbonization process, which may be caused by the curve of graphite layers. We can also find that the value of La decreases with the increase of lignin content, which further validates the inhibitory effect of lignin on the crystallization of AC. The results of the XRD and Raman spectroscopy analyses are shown in Table 2.
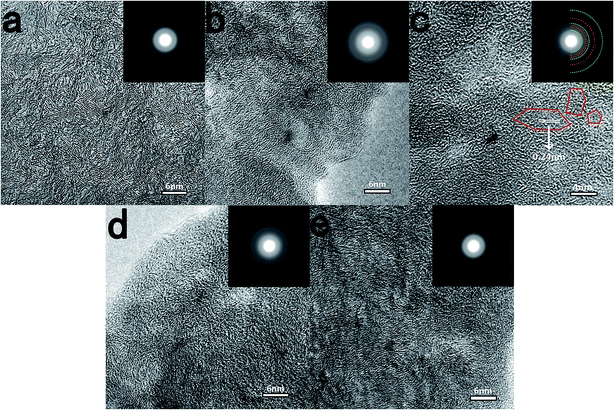
Fig. 2 shows HRTEM images of different ACs. There is no obvious long-range ordered structure in all ACs, revealing the amorphous nature of AC, which stands in sharp contrast to the highly perfect graphitization structure of pitch derived carbon (Fig. 2f). With the increase of carbonization temperature, the locally ordered structure develops further in a random direction over the entire area, especially for the AC111600 sample. It is clearly observed with HRTEM that some parallel carbon hexagonal layers and a large number of closed voids appear in the AC111600 sample, which should be due to the layer shift and fold with respect to each other. All selected area electron diffraction (SAED) patterns show dispersing diffraction rings, which is another demonstration of the amorphous structure of AC. The sharpness of diffraction rings increases with increasing HTTs, a further indication of the development of the graphitic structure. Furthermore, with increasing lignin content, the distance between parallel carbon hexagonal layers becomes larger, exhibiting a more disordered amorphous structure. This can also be proved by the gradually blurred diffraction rings in SAED patterns. The results of TEM and SAED further demonstrate that the lignin can suppress graphitization and improve the disordered degree of AC, which is favourable for Na storage.
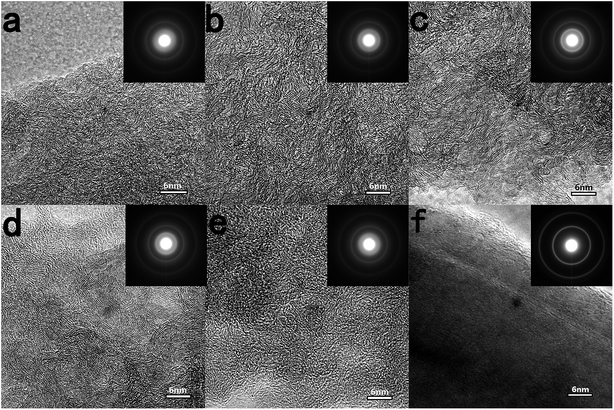 | ||
| Fig. 2 TEM images and SAED patterns of (a) AC111200, (b) AC111400, (c) AC111600, (d) AC731400, (e) AC371400 and (f) pitch derived carbon with HTTs at 1400 °C. | ||
In order to demonstrate that the emulsification interaction between pitch and lignin occurs in the preparation process of AC, we tested the carbon yield of pitch, several kinds of amorphous carbon precursors and the mixture of pitch and them by means of thermogravimetric analysis (TGA). The results of TGA are presented in Fig. 3a and S1.† The carbon yield of sucrose, lignin and phenolic resin at 1000 °C is 7%, 43% and 47%, respectively, while the value of pitch reaches up to 56%. When we mix pitch with sucrose, lignin and phenolic resin in a mass ratio of 1/1, the carbon yield of pitch/sucrose, pitch/lignin and pitch/phenolic resin is 38%, 57% and 52%, respectively. The result indicates that pitch/sucrose and pitch/phenolic resin are just physical mixtures while there is interaction between pitch and lignin in the pitch/lignin mixture. The emulsification interaction between pitch and lignin suppresses the carbon loss of the two in the high-temperature carbonization process, which ultimately results in higher carbon yield of pitch/lignin than that of pitch.
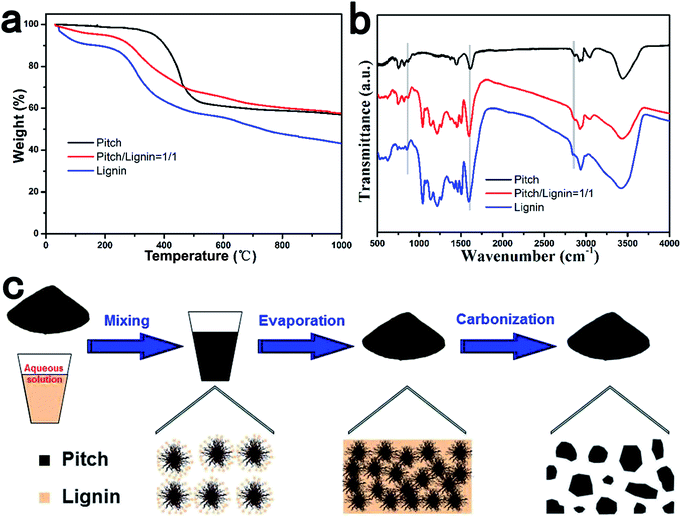 | ||
| Fig. 3 (a) TGA data and (b) FTIR spectra of pitch, lignin and the mixture of pitch and lignin with a weight ratio of 1/1; (c) principle schematic of the synthesis and proposed mechanism. | ||
FTIR spectra are also applied to evaluate the interaction between pitch and lignin in the pitch/lignin mixture, the result is shown in Fig. 3b. We can see four kinds of chemical bonds owned by both pitch and lignin: the OH stretching peak around 3430 cm−1, C–H asymmetric and symmetric stretching of methyl and methylene groups (2800–3100 cm−1), aromatic skeletal vibrations (1600 and 1458 cm−1) and C–H bending vibration in the benzene ring (670–870 cm−1). It is found that both the C–H stretching peak of methyl and methylene groups at 2840 cm−1 and the bending vibration peak of the benzene ring at 855 cm−1 of lignin move to higher wavenumber for the pitch/lignin mixture, which means that the existence of the interaction between the hydrogen atom of lignin and some atomic groups of pitch leads to the increase of stretching or bending vibration energy. This emulsification interaction can also be proved by the movement of the aromatic skeletal vibration peak at 1614 cm−1 of pitch to lower wavenumber which is caused by the increase of aromatic skeletal vibration energy. Fig. 3c presents a schematic of the proposed interaction mechanism between pitch and lignin. The clusters of pitch are surrounded by the molecules of lignin and the hydrophilic carboxyl groups arrange outside, thus pitch can be suspended in water forming emulsion with lignin. The result of FTIR spectroscopy successfully proves the existence of the emulsification interaction between pitch and lignin, explaining the TGA result.
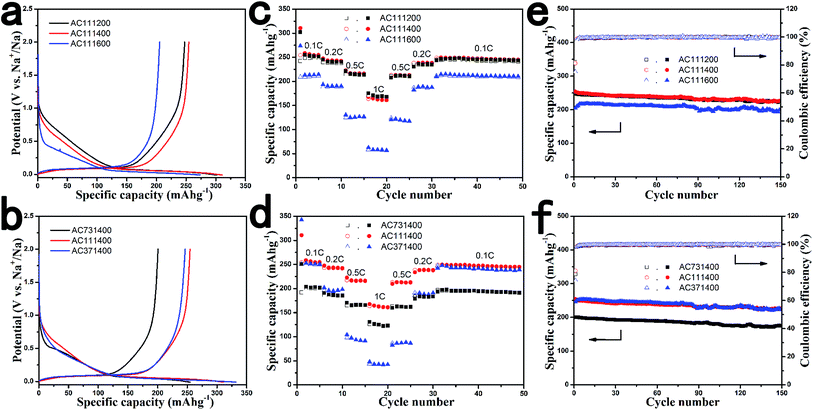
The electrochemical properties of AC under different conditions were first investigated using a half-cell test with a sodium foil as the counter electrode in order to further understand the influence of the material microstructure on Na storage behavior. The first galvanostatic discharge/charge curves at a current rate of 0.1C (30 mA g−1) are shown in Fig. 4a and b. The voltage profiles of all AC electrodes exhibit two distinct regions; (1) a gradual voltage decay around 2–0.115 V and (2) a plateau around 0.1 V. We can see the Na deposition potential at −0.015 V with an obvious turning point (Fig. S3a†). The AC111400 sample shows the highest reversible capacity of 254 mA h g−1 with a highest initial coulombic efficiency of 82% which is contributed by the low BET surface areas (Table 2), and the capacity percentage of the plateau region is about 65%. For comparison, the pitch derived carbon delivers a very low capacity of 85 mA h g−1 with an only sloping voltage profile (Fig. S2a and S3b†), which is consistent with its highly ordered structure. The reversible capacity and the plateau ratio in the overall capacity of the AC111200 sample and AC111600 sample are approximately 247 mA h g−1, 60% and 205 mA h g−1, 71%, and the initial coulombic efficiency of the AC111600 sample is as low as 74%. According to previous reports, the slope should correspond to Na storage in the defect sites, edges and the surface of graphene, and the plateau can be attributed to Na storage in the closed voids.43,44 The low capacity of the AC111600 sample could be ascribed to the less defects and the higher degree of crystallization which is not beneficial to Na storage. The large BET surface areas of the AC111600 sample significantly increase the formation of the SEI, resulting in the lowest initial coulombic efficiency. With the improvement of HTTs, the decreasing defects are disadvantageous to the storage of Na while the closed voids become relatively advantageous to Na storage, thus the plateau ratio in overall capacity increases with the increase of carbonization temperature.
The AC anodes with different lignin contents at the same HTTs display similar voltage profiles but there are some differences in capacity and the initial coulombic efficiency. Both the AC731400 sample and AC371400 sample have lower values than the AC111400 sample either in the Na storage capacity or in the initial coulombic efficiency, which could be attributed to variations of the microstructure. On one hand, the low capacity of the AC731400 sample is due to the high graphitization structure reducing available Na storage sites. On the other hand, the large BET surface areas of the AC371400 sample induced by increasing the lignin content result in irreversible capacity loss. All the above results show that the microstructure of amorphous carbon materials has a significant impact on the Na insertion and extraction capacity. The AC111400 sample exhibits the best performance in terms of high reversible capacity and initial coulombic efficiency as well as cost, which is on account of its appropriate microstructure combining both the defects and the closed voids beneficial to Na storage.
The rate performance of AC electrodes was investigated in detail at various rates from 0.1C to 1C to further identify the effects of HTTs and lignin content on the electrochemical performance, the results are shown in Fig. 4c and d. It can be seen that the rate performance deteriorates with increasing HTTs and the capacity decay at high rates is mainly due to the rapid capacity fading of the plateau region, especially for the AC111600 sample. This illustrates that the defect sites are more favourable for Na+ ion transport and the capacity retention of electrodes during large current operation than the closed voids, corresponding to previous reports.45 In addition, with the increase of lignin content, the decreasing electronic conductivity resulting from the more disordered graphitization structure is not beneficial to enhancing the rate performance of carbonaceous anode materials. Considering both electronic conductivity and ionic conductivity, the AC111400 sample shows outstanding rate performance with specific capacities of 212 mA h g−1 and 162 mA h g−1 at 0.5C and 1C, respectively. The capacity can return to the previous values when the current rate is reduced, suggesting a good stability of AC in a wide current range.
Fig. 4e and f show the cycling performance of AC under different conditions at 0.1C for 150 cycles. All five amorphous carbon materials have comparable cycling stability, indicating that the lignin content and carbonization temperature do not significantly impact the cycling performance. The AC111400 sample retains a capacity of 226 mA h g−1 after 150 cycles corresponding to a capacity retention of 89%, and the coulombic efficiencies can remain at nearly 100% after the first few cycles. Such excellent cycling performance is most likely due to the good mechanical properties and high electronic conductivity of the unique amorphous carbon material.
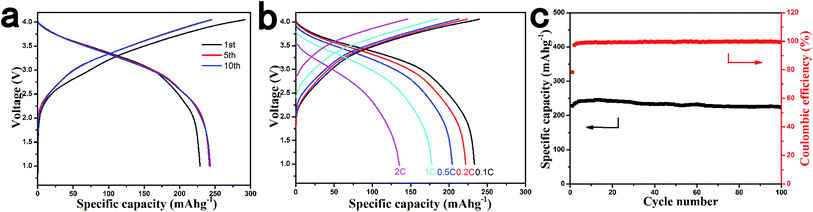
To further deepen the understanding of the Na storage mechanism in amorphous carbon materials, we carried out ex situ TEM experiments using the AC111400 electrode. The structural evolution of amorphous carbon in the discharge/charge process is shown in Fig. 5. When the AC111400 electrode is discharged to 0.115 V which is the cut-off voltage of the sloping capacity, the structure becomes more disordered, indicating Na storage on the surface of graphene. With further discharging to the final state, several Na metal nanoclusters are directly observed in the TEM image. The SAED pattern in the inset of Fig. 5c also clearly shows the appearance of (110), (111) and (220) crystal planes of Na metal after complete sodiation. Both TEM and SAED demonstrate the Na storage in closed voids of amorphous carbon, and the storage capacity corresponds to the plateau region in electrochemical curves, indicating closed voids provide more energetically stable sodium environments. The result also illustrates why there is no plateau region in the electrochemical curves of pitch derived carbon with a highly ordered graphitization structure. The structure of the AC111400 sample becomes more disordered after a cycle which ensures the cycling stability in the subsequent electrochemical cycle.
The AC111400 sample was coupled with a cathode of SIBs to demonstrate the application prospects of AC in the full cell. The O3–Na0.9[Cu0.22Fe0.30Mn0.48]O2 which is designed and prepared by our group for the first time was chosen as the cathode to assemble a full cell.20 The Na0.9[Cu0.22Fe0.30Mn0.48]O2 is of particular interest because of the excellent stability in air and even in water, and it is the only stable O3 layered oxide reported so far. In addition, the Na0.9[Cu0.22Fe0.30Mn0.48]O2 does not contain toxic and expensive transition metals, which makes it very suitable for practical application in grid electrical energy storage with only environmentally friendly and low-cost elements. The Na storage behaviour of Na0.9[Cu0.22Fe0.30Mn0.48]O2 is shown in Fig. S3,† stable cycle performance is achieved with a reversible capacity of 100 mA h g−1 at a current density of 10 mA g−1.
The preliminary electrochemical measurement results of full cells with the Na0.9[Cu0.22Fe0.30Mn0.48]O2 cathode and the AC111400 anode are displayed in Fig. 6. The Na0.9[Cu0.22Fe0.30Mn0.48]O2/AC111400 full cell delivers a reversible capacity of 240 mA h g−1 (based on the anode) after several cycle activation, an average operating voltage of 3.2 V and a high initial coulombic efficiency of 78% at a current rate of 0.2C (60 mA g−1). Even at a current rate of 1C, the full cell can still deliver approximately 177 mA h g−1. A superior cycle performance is shown in Fig. 6c with a capacity retention of 97% after 100 cycles. The energy density of this system is calculated to be 207 W h kg−1. In addition, to further reduce the cost and improve the energy density of the full cell, we used the cheaper and lighter Al foil instead of Cu foil as the current collector. A more environmentally benign aqueous sodium alginate binder was also used to reduce the environmental pollution brought by this system.
Conclusions
This work reported a novel high yielding and low-cost amorphous carbon anode material from pitch with an adjustable microstructure for sodium-ion batteries. Several amorphous carbon materials were first successfully fabricated from a mixture of pitch and lignin, and then the Na storage behaviour was investigated. The results of TGA and FTIR show that there is emulsification interaction between pitch and lignin, resulting in an extraordinary high carbon yield of 57%. The microstructure of AC varies with the variation of HTTs and the mass ratios of pitch to lignin, which leads to different electrochemical performances in SIBs depending on the defects and the closed voids. Among all ACs, the AC111400 sample shows the best overall performances with a reversible capacity of 254 mA h g−1, a high initial coulombic efficiency of 82% and good rate performance. The practical feasibility of the AC111400 sample in the full cell was further confirmed by combining with an air-stable O3–Na0.9[Cu0.22Fe0.30Mn0.48]O2 cathode, delivering an energy density of 207 W h kg−1 and excellent cycling stability with a capacity retention of 97% after 100 cycles. The results of this study show that tailoring a carbon microstructure is a critical factor for tuning the electrochemical performance of carbonaceous anode materials for SIBs. This work not only reports a superior low-cost amorphous carbon anode for SIBs with a promising application prospect but also offers a strategy for various electrode material designs in the future.Author contributions
Y.-S. H. conceived and designed this work; Y. M. L. performed all the synthesis and electrochemical experiments; Y. M. L. and Y.-S. H. wrote the paper; all the authors participated in analysis of the experimental data and discussions of the results as well as preparing the paper.Acknowledgements
This work was supported by funding from the NSFC (51222210, 11234013, and 51421002), “973” Projects (2012CB932900), and the One Hundred Talent Project of the Chinese Academy of Sciences.Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- L. M. Suo, Y.-S. Hu, H. Li, M. Armand and L. Q. Chen, Nat. Commun., 2013, 4, 1481 CrossRef PubMed.
- H. L. Pan, Y.-S. Hu and L. Q. Chen, Energy Environ. Sci., 2013, 6, 2338–2360 CAS.
- J. M. Tarascon, Nat. Chem., 2010, 2, 510 CrossRef CAS PubMed.
- S.-W. Kim, D.-H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710 CrossRef CAS.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947 CrossRef CAS.
- N. Yabuuchihttp, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636 CrossRef PubMed.
- S. Yuan, X.-L. Huang, D.-L. Ma, H.-G. Wang, F.-Z. Meng and X.-B. Zhang, Adv. Mater., 2014, 26, 2273 CrossRef CAS PubMed.
- N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada and S. Komaba, Nat. Mater., 2012, 11, 512 CrossRef CAS PubMed.
- D. D. Yuan, W. He, F. Pei, F. Y. Wu, Y. Wu, J. F. Qian, Y. L. Cao, X. P. Ai and H. X. Yang, J. Mater. Chem. A, 2013, 1, 3895 CAS.
- J. Xu, D. H. Lee, R. J. Clément, X. Yu, M. Leskes, A. J. Pell, G. Pintacuda, X. Q. Yang, C. P. Grey and Y. S. Meng, Chem. Mater., 2014, 26, 1260 CrossRef CAS.
- P. Vassilaras, A. J. Toumar and G. Ceder, Electrochem. Commun., 2014, 38, 79 CrossRef CAS.
- H. Yu, S. Guo, Y. Zhu, M. Ishida and H. Zhou, Chem. Commun., 2014, 50, 457 RSC.
- Y. S. Wang, J. Liu, B. Lee, R. M. Qiao, Z. Z. Yang, S. Y. Xu, X. Q. Yu, L. Gu, Y.-S. Hu, W. L. Yang, K. Kang, H. Li, X.-Q. Yang, L. Q. Chen and X. J. Huang, Nat. Commun., 2015, 6, 6401 CrossRef CAS PubMed.
- S. Y. Xu, Y. S. Wang, L. B. Ben, Y. C. Lyu, N. N. Song, Z. Z. Yang, Y. M. Li, L. Q. Mu, H. T. Yang, L. Gu, Y.-S. Hu, H. Li, Z.-H. Cheng, L. Q. Chen and X. J. Huang, Adv. Energy Mater., 2015, 5, 1501156 Search PubMed.
- K. T. Lee, T. N. Ramesh, F. Nan, G. Botton and L. F. Nazar, Chem. Mater., 2011, 23, 3593 CrossRef CAS.
- Z. L. Jian, W. Z. Han, X. Lu, H. X. Yang, Y.-S. Hu, J. Zhou, Z. B. Zhou, J. Q. Li, W. Chen, D. F. Chen and L. Q. Chen, Adv. Energy Mater., 2013, 3, 156 CrossRef CAS.
- P. Barpanda, G. Oyama, S.-I. Nishimura, S.-C. Chung and A. Yamada, Nat. Commun., 2014, 5, 4358 CAS.
- Y. M. Li, Z. Z. Yang, S. Y. Xu, L. Q. Mu, L. Gu, Y.-S. Hu, H. Li and L. Q. Chen, Adv. Sci., 2015, 2, 1500031 Search PubMed.
- L. Q. Mu, S. Y. Xu, Y. M. Li, Y.-S. Hu, H. Li, L. Q. Chen and X. J. Huang, Adv. Mater., 2015, 27, 6928–6933 CAS.
- P. Ge and M. Fouletier, Solid State Ionics, 1988, 28, 1172–1175 CrossRef.
- J. Liu, Y. Wen, P. A. van Aken, J. Maier and Y. Yu, Nano Lett., 2014, 14, 6387–6392 CrossRef CAS PubMed.
- J. F. Qian, Y. Chen, L. Wu, Y. L. Cao, X. P. Ai and H. X. Yang, Chem. Commun., 2012, 48, 7070 RSC.
- Y. H. Xu, Y. J. Zhu, Y. H. Liu and C. S. Wang, Adv. Energy Mater., 2013, 3, 128 CrossRef CAS.
- L. F. Xiao, Y. L. Cao, J. Xiao, W. Wang, L. Kovarik, Z. M. Nie and J. Liu, Chem. Commun., 2012, 48, 3321 RSC.
- V. L. Chevrier and G. Ceder, J. Electrochem. Soc., 2011, 15, A1011–A1014 CrossRef.
- H. L. Pan, X. Lu, X. Q. Yu, Y.-S. Hu, H. Li, X. Q. Yang and L. Q. Chen, Adv. Energy Mater., 2013, 3, 1186 CrossRef CAS.
- Y. Sun, L. Zhao, H. L. Pan, X. Lu, L. Gu, Y.-S. Hu, H. Li, M. Armand, Y. Ikuhara, L. Q. Chen and X. J. Huang, Nat. Commun., 2013, 4, 1870 CrossRef PubMed.
- X. Q. Yu, H. L. Pan, W. Wan, C. Ma, J. M. Bai, Q. P. Meng, S. N. Ehrlich, Y.-S. Hu and X.-Q. Yang, Nano Lett., 2013, 13, 4721 CrossRef CAS PubMed.
- Y. S. Wang, X. Q. Yu, S. Y. Xu, J. M. Bai, R. J. Xiao, Y.-S. Hu, H. Li, X. Q. Yang, L. Q. Chen and X. J. Huang, Nat. Commun., 2013, 4, 2365 Search PubMed.
- L. Zhao, J. M. Zhao, Y. S. Hu, H. Li, Z. B. Zhou, M. Armand and L. Q. Chen, Adv. Energy Mater., 2012, 2, 962–965 CrossRef CAS.
- Y. Wen, K. He, Y. J. Zhu, F. D. Han, Y. H. Xu, I. Matsuda, Y. Ishii, J. Cumings and C. S. Wang, Nat. Commun., 2014, 5, 4033 CAS.
- B. Jache and P. Adelhelm, Angew. Chem., Int. Ed., 2014, 53, 10169–10173 CrossRef CAS PubMed.
- H. Kim, J. Hong, Y.-U. Park, J. Kim, I. Hwang and K. Kang, Adv. Funct. Mater., 2015, 25, 534–541 CrossRef CAS.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 1271–1273 CrossRef CAS.
- S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, A. Ogata, K. Gotoh and K. Fujiwara, Adv. Funct. Mater., 2011, 21, 3859–3867 CrossRef CAS.
- K. Tang, L. J. Fu, R. J. White, L. H. Yu, M. M. Titirici, M. Antonietti and J. Maier, Adv. Energy Mater., 2012, 2, 873–877 CrossRef CAS.
- H.-G. Wang, Z. Wu, F.-L. Meng, D.-L. Ma, X.-L. Huang, L.-M. Wang and X.-B. Zhang, ChemSusChem, 2013, 6, 56 CrossRef PubMed.
- L. J. Fu, K. Tang, K. P. Song, P. A. van Aken, Y. Yu and J. Maier, Nanoscale, 2014, 6, 1384–1389 RSC.
- S. Wenzel, T. Hara, J. Janek and P. Adelhelm, Energy Environ. Sci., 2011, 4, 3342–3345 CAS.
- Y. M. Li, L. Q. Mu, Y.-S. Hu, H. Li, L. Q. Chen and X. J. Huang, Energy Storage Materials, 2015 DOI:10.1016/j.ensm.2015.10.003.
- L. Qie, W. M. Chen, H. H. Xu, X. Q. Xiong, Y. Jiang, F. Zou, X. L. Hu, Y. Xin, Z. L. Zhang and Y. H. Huang, Energy Environ. Sci., 2013, 6, 2497–2505 Search PubMed.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 4428–4431 CrossRef CAS.
- C. Bommier, T. W. Surta, M. Dolgos and X. L. Ji, Nano Lett., 2015, 15, 5888–5892 CrossRef CAS PubMed.
- Y. M. Li, S. Y. Xu, X. Y. Wu, J. Z. Yu, Y. S. Wang, Y.-S. Hu, H. Li, L. Q. Chen and X. J. Huang, J. Mater. Chem. A, 2015, 3, 71 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ta08601a |
| This journal is © The Royal Society of Chemistry 2016 |