Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication of a mercaptoacetic acid pillar[5]arene assembled nanochannel: a biomimetic gate for mercury poisoning†
Fan
Zhang
a,
Junkai
Ma
a,
Yue
Sun
a,
Imene
Boussouar
a,
Demei
Tian
a,
Haibing
Li
*ab and
Lei
Jiang
b
aKey Laboratory of Pesticide and Chemical Biology (CCNU), Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, P. R. China. E-mail: lhbing@mail.ccnu.edu.cn
bBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China
First published on 29th January 2016
Abstract
Mercury ion binding blocks potassium ion channels, which leads to toxicity in vivo. It is challenging to design a simple and efficient artificial system to mimic the sophisticated biological process of mercury poisoning. Herein, based on biomimetic strategies, a tunable mercury(II) ion-gate modulated by mercaptoacetic acid-pillar[5]arene (MAP5) is reported. By virtue of the unique design of the host–guest competition, potassium ion transport can actualize the reversible switching between “on” and “off” in the absence and presence of mercury ions. Moreover, the MAP5-immobilized nanochannel is highly effective at distinguishing Hg2+ from other metal ions and can be used to detect Hg2+ and act as an excellent and robust gate valve for developing integrated circuits and nanoelectronic logic devices. This study paves a new way for better understanding the physiological phenomenon of mercury toxicity and shows great promise for biomedical research.
Introduction
The mercury(II) ion is a highly toxic pollutant that can cause serious damage to nervous tissues and organs, such as lung damage, deterioration of the brain and kidneys, and so forth.1–6 Recently, a number of studies have demonstrated that mercury ion binding blocks potassium ion channels, which leads to toxicity in vivo.7–9 The biological process is complicated, which is quite significant when investigating the pathology and toxicology. Therefore, it is hugely challenging to fabricate a simple and efficient artificial device to mimic the biological process of mercury(II) ion gated potassium ion channels.The gating of biological ion channels is the basis of cellular signal-transduction processes.10,11 It always plays a crucial role in regulating the functionalities of biological channels, such as in opening and closing the ion channel, governing specific ion diffusion, and controlling ion conduction in response to specific stimuli.12–15 However, most of the biological channels in nature, embedded in lipid bilayers, are not stable and sophisticated.16,17 To better understand the complicated process of biological ion transport, artificial biomimetic nanochannels have been widely developed because of their excellent mechanical robustness and chemical stability.18–22 These solid-state synthetic nanochannels possess great flexibility in terms of their geometry and size, and have multi-functional surface properties.23–26 Especially the conical nanochannel in polyethylene terephthalate (PET), which has strong implications for the simulation of the different ionic transport processes as well as the enhancement of the functionality of biological ion channels.27–30 Recently, interest in nanochannels has been stimulated by discoveries of the importance of biological channels in many of the physiological processes of living organisms as well as in building functional gates. Hence, preparing nanochannels as smart switchable gates to mimic mercury ions poisoning has drawn enormous research attention.
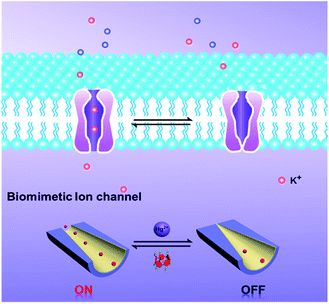
To the best of our knowledge, among the various methods for constructing switchable gates, host–guest binding and release can be used as a simple and robust method to fabricate “on–off” switches. Pillar[5]arenes, as a new type of macrocyclic host, have gained increasing attention in recent years.31–33 They have been used extensively as supramolecular switches for host–guest interactions, such as with pseudorotaxanes, rotaxanes, catenanes, supramolecular dimers, and so forth.34–36 With these in mind, we developed a new strategy to introduce molecular switches into nanochannel systems to fabricate a tunable mercury ion-gated nanochannel. Herein, inspired by the phenomenon of mercury ions binding with thiol containing protein blocks in the potassium ion channels, we designed and synthesized a water-soluble mercaptoacetic acid-pillar[5]arene (MAP5) using the “thiol–ene” click reaction. By virtue of host–guest interactions, MAP5 can assemble into the inner wall of the nanochannel which is modified with suitable guest molecules. Because mercury ions bind to thiol-containing molecules, mercury ions can remove MAP5 from the host–guest complex and change the surface charge and wettability of the nanochannel. Such a tunable mercury(II) ion gate with good molecular responsive properties can open and close in response to external stimuli and control potassium ion transport in the channels (Scheme 1).
Results and discussion
Synthesis of mercaptoacetic acid-pillar[5]arene
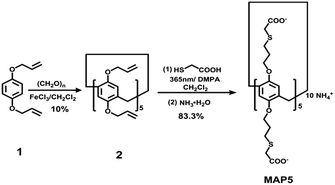
To obtain a Hg2+ responsive supramolecular switch that can be assembled in water, we designed and synthesized water-soluble mercaptoacetic acid-pillar[5]arene (MAP5) using a rapid photocatalytic click reaction. The strategy for MAP5 synthesis is shown in Fig. 1. Paraformaldehyde (0.9 g, 30 mmol) was added to a solution of monomer 1 (1.9 g, 10 mmol) in dichloromethane (30 mL) under a nitrogen atmosphere. Iron(III) chloride (FeCl3, 0.325 g, 2 mmol) was then added to the solution and the mixture was stirred at room temperature for 30 min. After the solvent was removed, the obtained solid was purified using column chromatography on silica gel with petroleum ether/ethyl acetate (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the eluent to obtain white powder 2 (0.2 g, 10% yield). Subsequently, MAP5 was synthesized using the classical “thiol–ene” click reaction. Mercaptoacetic acid (0.736 g, 8 mmol) and the photoinitiator 2,2-dimethoxy-2-phenylacetophenone (DMPA, 50 mg) were added to compound 2 (0.2 g, 0.2 mmol) in dichloromethane (20 mL) and exposed to 365 nm UV light under stirring at room temperature for 15 min. After solvent evaporation, the crude product was purified using column chromatography to give a white powder. Then the product was mixed with 40% NH3·H2O (10.0 mL) and stirred at reflux for 5 h. The mixture was concentrated under reduced pressure to obtain the precipitated product. The product was collected by filtration, washed with ethanol, and dried under vacuum to obtain MAP5 as a white solid (0.214 g, 83.3% yield), which was characterized using 1H NMR and 13C NMR spectroscopy, and MALDI-TOF (see Fig. S6–S8†).
1 v/v) as the eluent to obtain white powder 2 (0.2 g, 10% yield). Subsequently, MAP5 was synthesized using the classical “thiol–ene” click reaction. Mercaptoacetic acid (0.736 g, 8 mmol) and the photoinitiator 2,2-dimethoxy-2-phenylacetophenone (DMPA, 50 mg) were added to compound 2 (0.2 g, 0.2 mmol) in dichloromethane (20 mL) and exposed to 365 nm UV light under stirring at room temperature for 15 min. After solvent evaporation, the crude product was purified using column chromatography to give a white powder. Then the product was mixed with 40% NH3·H2O (10.0 mL) and stirred at reflux for 5 h. The mixture was concentrated under reduced pressure to obtain the precipitated product. The product was collected by filtration, washed with ethanol, and dried under vacuum to obtain MAP5 as a white solid (0.214 g, 83.3% yield), which was characterized using 1H NMR and 13C NMR spectroscopy, and MALDI-TOF (see Fig. S6–S8†).
The 1H NMR analysis of the supramolecular switch
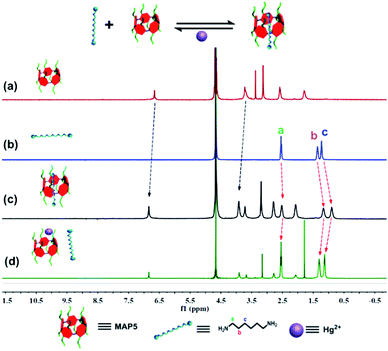
Based on molecular recognition, we devised a new strategy to introduce pillar[5]arene to the nanochannel using host–guest interactions. We chose 1,6-hexanediamine (HDA) as a model guest molecule immobilized on the surface. Initially, 1H NMR spectroscopy was performed to investigate the host–guest interaction of MAP5 and HDA. As shown in Fig. 2, when 1.0 equiv. of HDA was added to a solution of MAP5, chemical shift changes of some protons in HDA and MAP5 appeared (Fig. S9†). The protons Ha, Hb and Hc of the HDA alkyl chain exhibited substantial upfield shifts of Δδa = 0.062 ppm, Δδb = 0.164 ppm, and Δδc = 0.23 ppm, respectively, because of the inclusion-induced shielding effects when interacting with MAP5. Simultaneously, the proton of benzene in MAP5 was also downshifted by Δδ = 0.206 ppm. These shifts can be attributed to the alkyl chain being inserted into the cavity of MAP5 to form a host–guest complex. Details of the interaction of HDA and MAP5 were measured through 1H NMR titration. The mole ratio plots based on the proton NMR data showed that the complexes of MAP5 and HDA had a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry in water at room temperature (as shown in Fig. S10 and S11†). Moreover, computational calculations at the b3lyp/6-31G (d) levels verified the formation of a host–guest complex driven by hydrophobic interactions (Fig. S12†). When mercury(II) ions were introduced into the MAP5–HDA system, the chemical shift changes of HDA recovered because of the stronger affinity between Hg2+ and MAP5. On the other hand, the protons in MAP5 also exhibited slight chemical shift changes due to the interactions with Hg2+. These results revealed that Hg2+ successfully competes with HDA to form the MAP5–Hg2+ complex.
1 stoichiometry in water at room temperature (as shown in Fig. S10 and S11†). Moreover, computational calculations at the b3lyp/6-31G (d) levels verified the formation of a host–guest complex driven by hydrophobic interactions (Fig. S12†). When mercury(II) ions were introduced into the MAP5–HDA system, the chemical shift changes of HDA recovered because of the stronger affinity between Hg2+ and MAP5. On the other hand, the protons in MAP5 also exhibited slight chemical shift changes due to the interactions with Hg2+. These results revealed that Hg2+ successfully competes with HDA to form the MAP5–Hg2+ complex.
Construction of the supramolecular switch on the PET planar film
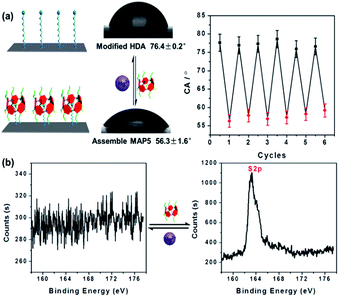
Encouraged by the above competition between Hg2+ and HDA in aqueous solution, we further investigated the tunable switch from the functionalized poly(ethylene terephthalate) (PET) membrane surface properties. In this experiment, to confirm that HDA and MAP5 modified the surface of the PET film successfully, the functionalized film was characterized using contact angle (CA) measurements and X-ray photoelectron spectroscopy (XPS) analysis. Fig. S14† shows that the CA of the etched membrane with its exposed –COO− groups is 61.9° ± 2.3°. After modification with HDA, the functionalized surface has a CA of 76.4° ± 0.2°. Then, when MAP5 attached to the HDA functionalized membrane by self-assembly, the membrane became more hydrophilic due to the multiple –COO− groups of MAP5 and had a CA of 54.7° ± 0.6°. Furthermore, XPS was used to evaluate the chemical composition of the PET membrane. Before modification (black line), the spectrum showed only a C 1s peak at 284.83 eV and an O 1s peak at 531.89 eV. Then the N 1s peak at 399.62 eV appears in the modified membrane owing to the nitrogen atom in HDA. The S 2p peak appears at 163.43 eV after MAP5 self-assembles on the surface of the PET membrane. Normally, the density of –COO− groups on the surface was estimated to be approximately 1 group per nm2.37 Therefore, according to the XPS derived relative content of N and S, we calculated the density of MAP5 modified on the surface to be approximately 0.01 MAP5 molecules per nm2 (see Fig. S15 and Tables S2–S4†).As wettability plays a crucial role in the switchable system of HDA–MAP5–Hg2+, we further investigated the wettability of the molecular switch using a HDA-modified PET planar film (Fig. 3a). The results show that before MAP5 assembled on the surface of the film, it appears to be hydrophilic with a CA of 76.4° ± 0.2°. The film surface containing MAP5, through the host–guest interaction with HDA, shows a significantly hydrophilic surface with a CA of 56.3° ± 1.6°. Upon addition of Hg2+, the CA returned to that of the HDA-modified film, which indicates that Hg2+ can remove MAP5 from the complex of HDA–MAP5 because of the high affinity between Hg2+ and MAP5. Moreover, the CA of the HDA film alternately immersed in MAP5 aqueous solution and Hg2+ aqueous solution could reversibly switch between 76.4 ± 0.2° and 56.3 ± 1.6°. Hence, according to these results, a cycling experiment of the HDA-modified PET surface in MAP5 and Hg2+ aqueous solutions was carried out. The CA switched six times, indicating the good reversible change of the wettability of the surface as a molecular switch. In addition, application of the HDA-modified PET surface as a molecular switch was also confirmed using XPS characterization. As shown in Fig. 3b, the S 2p peak at 163.43 eV is not present for the HDA-modified film, but when the HDA immobilized surface assembles with MAP5, the peak is clearly observed. After immersing the film in 1 mM Hg2+ solution and washing with deionized water, the S 2p peak disappears, which is caused by MAP5 being removed from the HDA film due to its stronger affinity for Hg2+. These results clearly demonstrate the excellent reversible properties of the HDA–MAP5 complex toward Hg2+.
 | ||
| Fig. 3 Reversible properties of the molecular switch on the PET planar film by (a) CA and (b) XPS characterization. It shows that the molecular switch can be well regulated by Hg2+ and MAP5. | ||
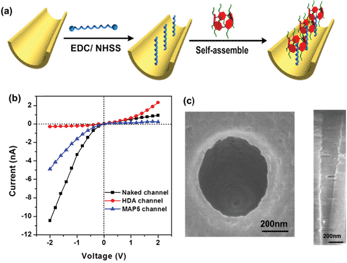
Fabrication of the MAP5 assembled nanochannel
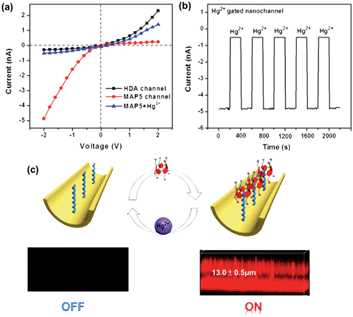
Based on the above properties in solution and on the PET surface, we attempted to fabricate a tunable mercury ion-gated nanochannel with the idea of the above supramolecular switch. Firstly, a single conical nanochannel was prepared using an asymmetric track-etch technique on a 12 μm thick PET membrane (Hostaphan RN12 Hoechst) with a single heavy-ion-irradiated track in the center. Before the etching process, each side of the PET membrane was exposed to UV light (365 nm) for 1 hour. Diameter measurements of the nanochannels were performed with a commonly used electrochemical method.38 The large opening (base) was about 560 nm in diameter and the tip diameter was calculated to be around 20 nm (see Fig. 4c and S17†). During the chemical etching process, carboxyl groups (–COO−) were exposed on the nanochannel surface, and then 1,6-diaminohexane (HDA) covalently linked to the –COO− groups by a classical EDC/NHSS cross-linking reaction. With the host–guest interaction, we fabricated the mercury(II) ion-gated system by adding a solution of MAP5 (1 mM) to the HDA-immobilized channel for self-assembly. After a further 1 hour, we removed the solution and washed the membrane with pure water three times. Ion current measurements were carried out using a Keithley 6487 picoammeter (Keithley Instruments, Cleveland, OH) in a custom-designed electrolyte cell, and the sample membrane was mounted between the two halves of the cell (see Fig. S16†). All of the experiments were carried out at room temperature (25 °C).The ion transport properties of the single nanochannel before and after MAP5 assembly were investigated by measuring current–voltage (I–V) curves with 0.1 M KCl solution as an electrolyte at a neutral pH value (pH 6.86) on both sides of the membrane. As shown in Fig. 4b, the original nanochannel showed a nonlinear I–V curve at neutral pH because of the presence of inherent anionic carboxylate groups (–COO−), which can preferentially transport cations (K+) from the tip entrance to the base side of the channel when a potential is applied across the membrane. After the chemical modification with HDA, there is an obvious decrease in the ionic current at −2 V and the immediate inversion of the rectifying characteristics indicate that successful modification by HDA resulted in a change in the polarity of the nanochannel from negative to positive (red line in Fig. 4b). Subsequently, MAP5 was attached to the nanochannel through self-assembly with HDA by the host–guest interaction. The rectifying characteristic returned to the initial state of cation (K+) selectivity due to the rich negative charges coming from MAP5.
The tunable properties of the mercury ion gated nanochannel
The tunable switch of this ion-gated nanochannel was evaluated by testing the current change with the addition of Hg2+ and MAP5. As shown in Fig. 5a, in the presence of MAP5, the surface of the nanochannel is hydrophilic and negatively charged, which leads to a high ion current and low resistance. Under these conditions, the gate shows the “on” state for potassium ion selective transport, and the current through the MAP5 assembled nanochannel is about −4.8 nA at a voltage of −2 V. After immersing the film in a solution of 1 mM Hg2+, the current at −2 V remarkably decreases to −0.5 nA because MAP5 is removed from the MAP5–HDA complexes. The pseudorotaxane complex transforms to the positively charged HDA-modified state, and the gate shows the “off” state for potassium ion transport inhibition. The HDA-modified nanochannel then recovers the function for binding MAP5. The reversibility of the ion current measured with the addition of MAP5 and Hg2+ could be repeated for six cycles. After several cycles, there was only a slight decrease in the ion current, indicating that the nanochannel is stable. Hence, the measurements confirm that this tunable system can be switched between the “on” and “off” states in response to MAP5 and Hg2+, and it can regulate potassium ion transport in the presence of Hg2+.To further demonstrate the reversibility of the nanochannel, laser scanning confocal microscopy was conducted. We used the fluorescent MAP5 derivative (MAP5–RhB), which was synthesized by linking the carboxyl group (–COO−) of MAP5 to rhodamine B amine (RhB–NH2). A host–guest complex was then formed on the HDA-modified porous PET membrane by the interaction between HDA and MAP5–RhB. As shown in Fig. 5c, when MAP5–RhB successfully assembles on the HDA immobilized nanochannel, a fluorescence signal appears (the “on” state). The fluorescence thickness is ca. 13.0 ± 0.5 μm, which agrees with the actual thickness of the PET membrane. When Hg2+ is added to the system, the fluorescence is significantly quenched (the “off” state) as the MAP5–RhB is removed from the nanochannel. Clearly, all the above data show that the MAP5 assembled nanochannel exhibits an excellent Hg2+ recognition capability and can act as an excellent model to mimic sophisticated physiological processes.
The ion selectivity of the MAP5 assembled nanochannel
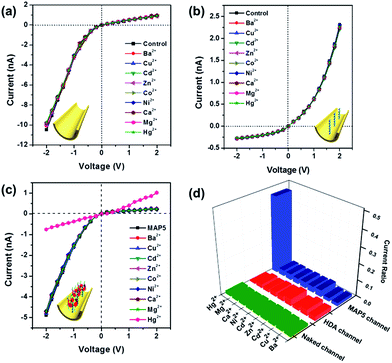
Additionally, the MAP5 assembled nanochannel could also have good Hg2+ selective gated properties. Fig. 6 shows I–V curves of the MAP5 assembled nanochannel exposed to electrolytes containing 10 μM Ba2+, Cu2+, Cd2+, Zn2+, Co2+, Ni2+, Ca2+, Mg2+, and Hg2+. The presence of Hg2+ results in a drastic decrease in the ion flux across the MAP5-assembled nanochannel, whereas the current is nearly constant in the presence of the other tested ions. This can be ascribed to the thiol of MAP5 having a stronger affinity for Hg2+ than the other ions. Thus, other metal ions cannot change the surface properties of the nanochannel. The current-change ratio ((I − I0)/I0) at −2 V was determined to quantify the changes in the ion current passing through the modified nanochannels in the presence of different metal ions. Compared with the current ratios from the naked channel and HDA channel, we found that only the MAP5 assembled channel can act as a good selectivity binding site for Hg2+. Moreover, the Hg2+-responsive properties were also confirmed using UV-vis spectroscopy (Fig. S13†), from which we found that only Hg2+ can enhance the characteristic peak. Therefore, these results indicate that Hg2+ can efficiently control the “off” and “on” states in the MAP5-modified nanochannel.To further illustrate the gating ratio of the mercury ion regulated nanochannel, the current–concentration (I–C) properties of the MAP5-assembled nanochannel for Hg2+ were determined. As shown in Fig. S18,† the transmembrane ion current at −2 V dramatically decreases with increasing Hg2+ concentration. With a Hg2+ concentration range from 1 nM to 1 μM (Fig. S18a†), the current gradually decreases, which indicates that the gate is closed. Therefore, the gating ratio can be defined as the current changing ratio. From the results, with 1 nM Hg2+ in the system, the potassium current is close to 6.5%. Moreover, when the concentration of Hg2+ is more than 1 μM, the current sharply decreases and there is gradual inversion of the I–V curve. This illustrates that Hg2+ can bind with MAP5 and form a stable MAP5–Hg2+ complex, gradually removing MAP5 from the HDA-modified nanochannel, which can act as a good gate for regulating and controlling potassium ion transport.
Conclusions
In summary, a biomimetic tunable mercury(II)-gated nanochannel was successfully developed by immobilizing MAP5 on a HDA-functionalized nanochannel. The system shows a responsive switching ability and stability by virtue of the unique host–guest interaction. This bioinspired ion gate systematically regulates potassium ion transport in the presence of mercury ions. When the channel shows the “on” state, potassium ion transport proceeds freely, representative of a normal biological organism; when it shows the “off” state, the potassium ion channel is blocked, indicating mercury poisoning. This work provides an interesting insight to comprehensively understand the important physiological process of mercury(II) ions binding with and blocking potassium ion channels, which results in toxicity. Moreover, the device easily distinguishes Hg2+ from other metal ions with a detection limit of 1 nM, which has potential applications in biosensors, toxicological testing for Hg2+, and as an excellent and robust gate valve for developing integrated circuits and programmable nanoelectronic logic devices.Experimental section
Nanochannel preparation
The single conical nanochannel was produced in a PET membrane (Hostaphan RN12 Hoechst, 12 μm thick, with single ion tracks in the center) using an asymmetric track-etched technique. Briefly, the PET membrane was embedded between the two chambers of a conductivity cell at about 35 °C. One chamber was filled with etching solution (9 M NaOH), while the other side was filled with stopping solution (1 M HCOOH + 1 M KCl) (Fig. S16†). For observation of the etching process, the voltage (1 V) used to monitor the etching process was applied in such a way that the transmembrane ion current could be observed as soon as the nanochannel opened. The etching process was stopped at a desired current value corresponding to a certain tip diameter. Then the membrane was soaked in MilliQ water (18.2 MΩ) to remove residual salts.Modification
As a result of the chemical etching, carboxyl groups are generated on the nanochannel surface. These can be activated with EDC/NHSS, forming an amine-reactive ester intermediate. Then these reactive esters were further condensed with 1,6-hexanediamine (HDA) through the formation of covalent bonds. In this paper NHSS ester was formed by soaking PET film in an aqueous solution of 15 mg of EDC and 3 mg of NHSS for 1 hour. After that the film was washed with distilled water and treated with 1 mM HDA solution overnight. Finally, the modified film was washed three times with distilled water. Then, mercaptoacetic acid-pillar[5]arene (MAP5) was attached to the HDA-channel through self-assembly.Ion current measurements
Ion currents were measured using a Keithley 6487 picoammeter (Keithley Instruments, Cleveland, OH). Ag/AgCl electrodes were used to apply a transmembrane potential across the film. The film was mounted between the two halves of a conductance cell. Both halves of the cell were filled with 0.1 M KCl, pH 6.86. In order to record the I–V curves, a scanning triangle voltage signal from −2 V to +2 V with a 40 s period was selected. Each test was repeated 5 times to obtain the average current value at different voltages.XPS
X-ray photoelectron spectra (XPS) data were obtained with an ESCALab220i-XL electron spectrometer from VG Scientific using 300 W Al Kα radiation. All peaks were referenced to C 1s (CHx) at 284.8 eV in the deconvoluted high resolution C 1s spectra.Contact angle measurements
Contact angles were measured using an OCA 20 contact angle system (Dataphysics, Germany) at 25 °C. Before the contact angle test, the sample was blown dry with N2. In each measurement, a droplet of about 2 μL of water was dispensed onto the surface of the PET membrane. The average contact angle value was obtained at five different positions of the same membrane.Confocal fluorescence images
Confocal images were acquired using a Zeiss confocal laser scanning unit mounted on an LSM710 fixed-stage upright microscope.Gaussian calculation
Computational calculations were carried out at the density functional theory b3lyp/6-31G (d) levels using Gaussian 03.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21572076, 21372092), Natural Science Foundation of Hubei Province (2013CFA112, 2014CFB246), Wuhan scientific and technological projects (2015020101010079) and the Self-determined research funds of CCNU from the colleges' basic research and operation of MOE (CCNU15KFY005). The authors thank Prof. Henry S. White for providing valuable suggestions.Notes and references
- D. W. Huang, C. G. Niu, M. Ruan, X. Y. Wang, G. M. Zeng and C. H. Deng, Environ. Sci. Technol., 2013, 47, 4392–4398 CrossRef CAS PubMed.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed.
- L. D. Hylander and M. E. Goodsite, Sci. Total Environ., 2006, 368, 352–370 CrossRef CAS PubMed.
- D. P. Wojcik, M. E. Godfrey, D. Christie and B. E. Haley, Neuroendocrinol. Lett., 2006, 27, 415–423 CAS.
- Y. K. Yang, S. K. Ko, I. Shin and J. Tae, Nat. Protoc., 2007, 2, 1740–1744 CrossRef CAS PubMed.
- T. W. Clarkson and L. Magos, Crit. Rev. Toxicol., 2006, 36, 609–662 CrossRef CAS PubMed.
- T. Narahashi, O. Arakawa, M. Nakahiro and D. A. Twombly, Ann. N. Y. Acad. Sci., 1991, 625, 26–36 CrossRef CAS PubMed.
- R. Ishikawa, Hokkaido Igaku Zasshi, 2003, 78, 479–483 CAS.
- J. Gutknecht, J. Membr. Biol., 1981, 61, 61–66 CrossRef CAS.
- (a) M. Y. Liu, H. C. Zhang, K. Li, L. P. Heng, S. T. Wang, Y. Tian and L. Jiang, Adv. Funct. Mater., 2015, 25, 421–426 CrossRef CAS; (b) M. Ali, S. Nasir, I. Ahmed, L. Fruk and W. Ensinger, Chem. Commun., 2013, 49, 8770–8772 RSC.
- Y. L. Shang, Y. Q. Zhang, P. Li, J. Lai, X. Y. Kong, W. D. Liu, K. Xiao, G. H. Xie, Y. Tian, L. P. Wen and L. Jiang, Chem. Commun., 2015, 51, 5979–5981 RSC.
- C. C. Harrell, P. Kohli, Z. Siwy and C. R. Martin, J. Am. Chem. Soc., 2004, 126, 15646–15647 CrossRef CAS PubMed.
- (a) H. C. Zhang, X. Hou, L. Zeng, F. Yang, L. Li, D. D. Yan, Y. Tian and L. Jiang, J. Am. Chem. Soc., 2013, 135, 16102–16110 CrossRef CAS PubMed; (b) H. C. Zhang, X. Hou, J. Hou, L. Zeng, Y. Tian, L. Li and L. Jiang, Adv. Funct. Mater., 2015, 25, 1102–1110 CrossRef CAS.
- (a) M. Ali, P. Ramirez, H. Q. Nguyen, S. Nasir, J. Cervera, S. Mafe and W. Ensinger, ACS Nano, 2012, 6, 3631–3640 CrossRef CAS PubMed; (b) H. C. Zhang, Y. Tian and L. Jiang, Chem. Commun., 2013, 49, 10048–10063 RSC.
- K. Xiao, G. Xie, P. Li, Q. Liu, G. L. Hou, Z. Zhang, J. Ma, Y. Tian, L. P. Wen and L. Jiang, Adv. Mater., 2014, 26, 6560–6565 CrossRef CAS PubMed.
- D. C. Gadsby, Nat. Rev. Mol. Cell Biol., 2009, 10, 344–352 CrossRef CAS PubMed.
- (a) B. Yameen, M. Ali, R. Neumann, W. Ensinger, W. Knoll and O. Azzaroni, Small, 2009, 5, 1287–1291 CrossRef CAS PubMed; (b) L. J. Gao, P. Li, Y. Q. Zhang, K. Xiao, J. Ma, G. H. Xie, G. L. Hou, Z. Zhang, L. P. Wen and L. Jiang, Small, 2015, 11, 543–547 CrossRef CAS PubMed.
- (a) E. Perozo, D. M. Cortes, P. Sompornpisut, A. Kloda and B. Martinac, Nature, 2002, 418, 942–948 CrossRef CAS PubMed; (b) B. Eisenberg, Acc. Chem. Res., 1998, 31, 117–123 CrossRef CAS.
- R. MacKinnon, Angew. Chem., Int. Ed., 2004, 43, 4265–4277 CrossRef CAS PubMed.
- J. Jasti, H. Furukawa, E. B. Gonzales and E. Gouaux, Nature, 2007, 449, 316–323 CrossRef CAS PubMed.
- D. C. Gadsby, Nat. Rev. Mol. Cell Biol., 2009, 10, 344–352 CrossRef CAS PubMed.
- L. Lin, J. Yan and J. Li, Anal. Chem., 2014, 86, 10546–10551 CrossRef CAS PubMed.
- (a) H. C. Zhang, X. Hou, Z. Yang, D. D. Yan, L. Li, Y. Tian, H. T. Wang and L. Jiang, Small, 2015, 11, 786–791 CrossRef CAS PubMed; (b) G. R. Nie, Y. Sun, F. Zhang, M. M. Song, D. M. Tian, L. Jiang and H. B. Li, Chem. Sci., 2015, 6, 5859–5865 RSC.
- S. C. Yang, Microfluid. Nanofluid., 2006, 2, 501–511 CrossRef CAS.
- Y. He, D. Gillespie, D. Boda, I. Vlassiouk, R. S. Eisenberg and Z. S. Siwy, J. Am. Chem. Soc., 2009, 131, 5194–5202 CrossRef CAS PubMed.
- (a) L. Yang, Q. F. Zhai, G. J. Li, H. Jiang, L. Han, J. H. Wang and E. K. Wang, Chem. Commun., 2013, 49, 11415–11417 RSC; (b) Y. Li, D. Wang, M. M. Kvetny, W. Brown, J. Liu and G. Wang, Chem. Sci., 2015, 6, 588–595 RSC.
- Z. J. Guo, J. H. Wang, J. T. Ren and E. K. Wang, Nanoscale., 2011, 3, 3767–3773 RSC.
- M. Ali, S. Nasir, P. Ramirez, J. Cervera, S. Mafe and W. Ensinger, ACS Nano, 2012, 6, 9247–9257 CrossRef CAS PubMed.
- S. Buchsbaum, G. Nguyen, S. Howorka and Z. S. Siwy, J. Am. Chem. Soc., 2014, 136, 9902–9905 CrossRef CAS PubMed.
- Z. S. Siwy, M. R. Powell, A. Petrov, E. Kalman, C. Trautmann and R. S. Eisenberg, Nano Lett., 2006, 8, 1729–1734 CrossRef PubMed.
- T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed.
- I. Nierengarten, M. Nothisen, D. Sigwalt, T. Biellmann, M. Holler, J. S. Remy and J. F. Nierengarten, Chem.–Eur. J., 2013, 19, 17552–17558 CrossRef CAS PubMed.
- W. Si, P. Y. Xin, Z. T. Li and J. L. Hou, Acc. Chem. Res., 2015, 48, 1612–1619 CrossRef CAS PubMed.
- (a) X. B. Hu, L. Chen, W. Si, Y. H. Yu and J. L. Hou, Chem. Commun., 2011, 47, 4694–4696 RSC; (b) P. J. Cragg and K. Sharma, Chem. Soc. Rev., 2012, 41, 597–607 RSC.
- (a) C. J. Li, L. Zhao, J. Li, X. Ding, S. H. Chen, Q. L. Zhang, Y. H. Yu and X. S. Jia, Chem. Commun., 2010, 46, 9016–9018 RSC; (b) M. Xue, Y. Yang, X. D. Chi, Z. B. Zhang and F. H. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed.
- (a) R. Klajn, L. Fang, A. Coskun, M. A. Olson, P. J. Wesson, J. F. Stoddart and B. A. Grzybowski, J. Am. Chem. Soc., 2009, 131, 4233–4235 CrossRef CAS PubMed; (b) A. Coskun, P. J. Wesson, R. Klajn, A. Trabolsi, L. Fang, M. A. Olson, S. K. Dey, B. A. Grzybowski and J. F. Stoddart, J. Am. Chem. Soc., 2010, 132, 4310–4320 CrossRef CAS PubMed.
- (a) I. Vlassiouk and Z. S. Siwy, Nano Lett., 2007, 7, 552–556 CrossRef CAS PubMed; (b) Y. He, D. Gillespie, D. Boda, I. Vlassiouk, R. S. Eisenberg and Z. S. Siwy, J. Am. Chem. Soc., 2009, 131, 5194–5202 CrossRef CAS PubMed.
- J. E. Wharton, P. Jin, L. T. Sexton, L. P. Horne, S. A. Sherrill, W. K. Mino and C. R. Martin, Small, 2007, 3, 1424–1430 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc04726a |
| This journal is © The Royal Society of Chemistry 2016 |