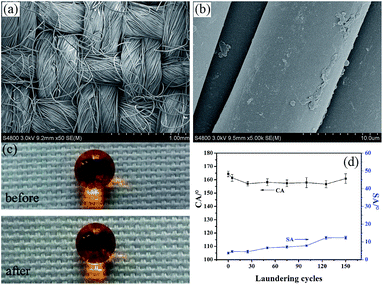
Fabrication of robust superhydrophobic fabrics based on coating with PVDF/PDMS
Chao-Hua Xue*ab,
Xing Lia,
Shun-Tian Jiab,
Xiao-Jing Guob and
Min Lib
aCollege of Chemistry & Chemical Engineering, Shaanxi University of Science and Technology, Xi'an 710021, China
bCollege of Resource & Environment, Shaanxi University of Science and Technology, Xi'an 710021, China. E-mail: xuech@zju.edu.cn
First published on 12th August 2016
Abstract
The functionalization of fabrics is important in both academic research and industrial areas. In this work, poly(ethylene terephthalate) (PET) fabrics were dipped into a solution of PVDF and PDMS in DMF/THF to coat fibers with PVDF/PDMS. Then, the as-coated fabrics with wet PVDF/PDMS were squeezed to remove the residual liquid with a scraping bar and put into a non-solvent water to induce roughening of the PVDF/PDMS coatings on the fibers, through a phase inversion process to obtain robust superhydrophobic PET fabrics. The obtained fabrics show a water contact angle of 164.4° ± 2.1° and a slide angle less than 4°. The superhydrophobicity of the fabrics was chemically stable in both acidic and alkaline solution, and could endure prolonged UV irradiation. In addition, after 5000 abrasion cycles or 150 times laundering, the coating still maintained superhydrophobicity and had a relatively low sliding angle. Also, the superhydrophobic fabrics displayed excellent self-cleaning properties. This simple process without using expensive equipment can be applied into the practical production of PET fabrics.
Introduction
Inspired by several natural phenomena, such as the self-cleaning phenomenon of the lotus leaf and the wings of butterflies, superhydrophobic surfaces with a water contact angle (CA) greater than 150° and a sliding angle (SA) less than 10° has attracted tremendous attention in academic research and industrial areas.1–12 Some of the emerging applications of these surfaces include anti-icing,13,14 corrosion-resistance,15,16 oil–water separation,17–19 microfluidics20 and other such applications.21,22 It is well known that micro-/nanostructure roughness and a low surface energy are essential characteristics for the preparation of superhydrophobic surfaces.23–29 Based on this principle, various techniques have been introduced to prepare superhydrophobic surfaces on different substrates.30–35 Among these artificial superhydrophobic surfaces, waterproof fabrics are considered to be the most promising ones.When rendering fabrics with the function of superhydrophobicity, the biggest issue is their low washing and abrasion durability. Therefore, it is necessary to improve their mechanical robustness for practical applications.36–40 Several strategies have been proposed to improve the mechanical robustness of superhydrophobic surfaces, such as creating multiscaled roughness on the substrate, introducing covalent bonding between the superhydrophobic coating and substrates, preparing a self-healing superhydrophobic surface and introducing elastomeric nanocomposites. For instance, durable superhydrophobic colorful textiles were prepared through chemical etching and roughening the poly(ethylene terephthalate) fiber surfaces, followed by diffusion of fluoroalkylsilane into the roughened fibers using a traditional textile dyeing machine, which showed strong durability against severe abrasion, prolonged laundering, and boiling water.41 Lin et al.42 prepared a self-healing and durable superamphiphobic fabric using a mixture consisting of poly(vinylidene fluoride-co-hexafluoropropylene), fluoroalkyl silane, and modified silica nanoparticles. The coated fabrics could withstand standard laundering for a long period of time and abrasion without apparently changing the superamphiphobicity. Also, the coating was very stable to strong acid/base, ozone, and boiling treatments and had a self-healing property for a short duration of heat treatment or room temperature ageing. Xue et al.43 reported on superhydrophobic PET fabrics which were prepared via thiol–ene click chemistry by establishing chemical bonds between the coating and substrate. The modified fabrics were resistant to UV irradiation, chemical etching, mechanical laundering and abrasion. Importantly, the surface of the superhydrophobic fabrics showed potential applications in water/oil separation. Zhou et al.44 fabricated a fluoroalkyl silane modified silicone rubber nanoparticle composite coating, which endowed fabrics with remarkable durability against strong acid, strong alkali, repeated machine washes, boiling water and severe abrasion damages, whilst retaining its superhydrophobicity.
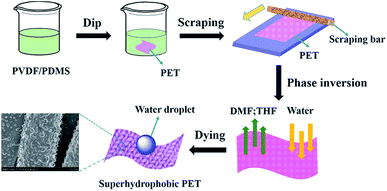
In this paper, we present a new method by using polyvinylidene fluoride (PVDF) and polydimethylsiloxane (PDMS) to fabricate superhydrophobic fabrics via a nonsolvent-induced phase-inversion process. This process can be described as a separating process, whereby the exchange of the DMF/THF solvent in the polymer solution with non-solvent water as the coagulation bath results in a rough morphology. The simple preparation process is shown in Fig. 1. The superhydrophobic fabrics showed excellent wear-resistance, laundering durability, UV resistance and pH stability. Also, the superhydrophobic fabrics displayed excellent self-cleaning properties.
Experimental
Materials
Polyvinylidene fluoride (PVDF) was purchased from Solvay. Polydimethylsiloxane (PDMS) precursor part-A (Sylgard 184 elastomer base) and precursor part-B (Sylgard 184 curing agent) were purchased from Dow Corning. PET fabrics were purchased from a local factory. Tetrahydrofuran (THF) and N,N-dimethylformamide (DMF) were purchased from Tianjin Fuyu Fine Chemical Co., Ltd. All of the chemicals were used as received without further purification.Preparation of superhydrophobic coating
PET fabric samples were cut into 15 × 20 cm and washed with deionized water to get rid of the impurities. 0.2 g of PVDF was dissolved in 19.8 g of DMF to form solution A, and 0.2 g of PDMS precursor part-A and 0.02 g of PDMS precursor part-B were dissolved in 19.78 g of THF to form solution B. Then, solution A and B were mixed to form a coating solution at room temperature. The PET fabrics were dipped into the coating solution, followed by the removal of the residual solution with a scraping bar. Subsequently, the coated fabrics were immersed in a water bath for 10 min to undergo phase inversion. The resulting fabrics were dried at 40 °C for 2 h. The as-obtained samples were denoted as PVDF/PDMS-P-PET. By comparison, the sample coated only by PVDF was denoted as PVDF-P-PET. The weight gain of the fabrics after being coated with the composite coating was determined according to the following equation:
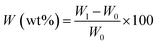 | (1) |
Characterization
The surface morphologies of the fibers were examined and the surface element compositions were analyzed using a Hitachi S-4800 field emission scanning electron microscope (SEM) with an energy dispersive X-ray. Samples were sputter-coated with gold prior to examination. Water contact angles (CA) and sliding angles (SA) of the fabrics were respectively determined with 5 μL and 20 μL deionized water droplets on a video optical contact angle system (OCA 20, DataPhysics, Germany) at ambient temperature. All the contact angles and sliding angles (SA) were determined by averaging values measured at five different points on each sample surface.Resistance evaluation of superhydrophobic PET fabrics
The abrasion resistance of the fabrics was evaluated by a modified procedure based on the AATCCA Test Method 8-2001 as reported previously.41 A pure nylon fabric cloth was used as the abrasion partner and the superhydrophobic fabric was fixed onto the stainless steel column with a loading pressure of 45 kPa. Then, the sample was subjected to a roundtrip with a distance of 200 mm, which is defined as one cycle.The washing durability of treated polyester fabrics was tested by a standard procedure according to AATCC Test Method 61-2003 Test No. 1A, as reported by our group.43 The samples were washed with a laundering machine (SW-12 AII, Da Rong, China) at 40 °C in the presence of 10 stainless steel balls along with 0.37 wt% soap powder. One washing cycle (45 min) is approximately equivalent to five commercial launderings. The washed fabrics were rinsed by abundant water to remove the residual detergent and dried at 80 °C.
The chemical durability of the superhydrophobicity was evaluated by immersing the samples into different pH solutions which was adjusted by hydrochloric acid or sodium hydroxide. The samples were rinsed and dried at 80 °C for CA measurements after immersion into the liquid for 48 h.
UV durability was conducted by irradiation using an artificial light source (UV lamp, Osram Ultra Vitalux 300 W) emitting a Gaussian-shaped spectrum which peaked at 370 nm with a cut off at 290 nm. Fabrics were placed under the UV lamp for continuous irradiation.
Results and discussion
Characterization of superhydrophobic fabrics
Scanning electron microscopy (SEM) was employed to characterize the surface morphologies of the pristine PET and the corresponding modified PET fibers. As shown in Fig. 2(a), it can be seen that the pristine fibers are smooth and round with a diameter about 11 μm which can provide the micro-structure of the fabrics. When treated by a single component of 1% PVDF (Fig. 2(b)), the fibers were decorated with PVDF aggregates with nanoscale roughness, which were induced by the non-solvent water exchange of the solvent DMF. However, the PVDF aggregate coating is very fragile to be eliminated due to the weak interaction between PVDF and PET. Therefore, we incorporated PDMS which has a good affinity with the PET fiber surfaces. Fig. 2(c) shows the morphology of the PVDF/PDMS coating on fibers after a phase-inversion process, in which water was used as a non-solvent for PVDF/PDMS. Therefore, phase separation would occur and the polymeric PVDF/PDMS preferred to precipitate to form aggregates on the fiber surface when the treated PET fabric was immersed into the water. Although the weight gain of the fabric after the composite coating was only about 0.8%, the coated fiber was completely covered with particulate protrusions and became much rougher. Combining with the microscale roughness of the fibers, a micro/nano-hierarchical structure was constructed, which is well known to enhance the hydrophobicity of the fabric. In combination with the low surface energy property of PVDF and PDMS itself, the superhydrophobic fabrics were successfully obtained. As a comparison, the PVDF/PDMS coated fabric without phase-inversion showed no roughness on the fiber surface except a slight roughening between the fiber gaps, as shown in Fig. 2(d). This confirmed the important effect of non-solvent induced phase-inversion on the fiber roughness, favoring the superhydrophobicity of the fabrics. | ||
| Fig. 2 SEM images of (a) pristine PET, (b) PVDF-P-PET, (c) PVDF/PDMS-P-PET, and (d) PVDF/PDMS coated PET fibers without phase-inversion. | ||
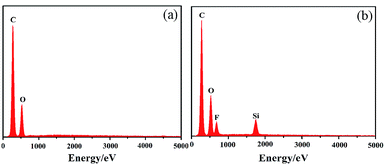
Meanwhile, energy dispersive spectroscopy (EDS) analysis had been conducted to examine the surface chemical composition of the fabric. As shown in Fig. 3(a), only peaks of C and O were detected on the pristine fabric and no other impurities could be observed. After the PVDF/PDMS treatment of the fabric, new peaks for F and Si were observed, as shown in Fig. 3(b). F is mainly from PVDF, while Si is from PDMS, indicating that the coating containing both PVDF and PDMS successfully covered the fabric.
Superhydrophobic property of fabrics
Fig. 4 shows the wettability of the pristine fabric and the superhydrophobic PET fabric. When a water droplet was dropped onto the samples, it easily spread and got the pristine fabric wet (Fig. 4(a)), which was due to the capillary effect of the fibrous structure. However, water droplets maintained a round shape on the PVDF/PDMS-P-PET coated fabric with a CA of 163.7° (Fig. 4(b)), showing typical superhydrophobicity. | ||
| Fig. 4 Digital images of water droplets after contact with the (a) pristine PET and (b) PVDF/PDMS-P-PET. The inset in (b) is an image of the contact angle. | ||
In order to further compare the wettability of the pristine fabric and the superhydrophobic PET fabric, we immersed the samples into water. The pristine fabric sunk to the bottom of the beaker, while the superhydrophobic PET fabric floated on the surface of water, and could also support a certain weight (Fig. 5(a)). When the superhydrophobic PET fabric was forced into the water by tweezers, the fabric surface appeared bright silver with a mirror-like phenomenon due to the existence of trapped air between water and the superhydrophobic surface (Fig. 5(b)). This trapped air could effectively prevent water from wetting the fabric.
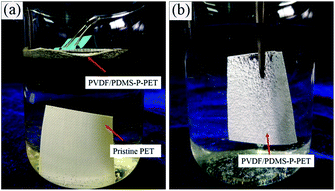 | ||
| Fig. 5 (a) Free immersion pristine PET and PVDF/PDMS-P-PET in the dyed water. (b) Immersion of PVDF/PDMS-P-PET fabric into the water by tweezers. | ||
Stability of the superhydrophobic fabrics
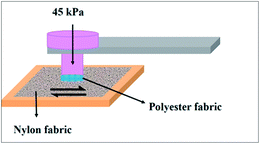
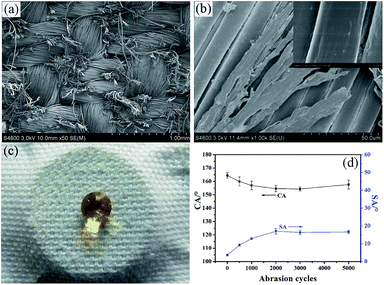
Taking practical applications into account, it is significant to evaluate the stability and the durability of the superhydrophobic fabric. The mechanical durability was examined by an abrasion test according to a modified procedure based on AATCCA Test Method 8-2001. Fig. 6 is the schematic of a friction instrument for fabrics. Briefly, the sample was subjected to a normal pressure (45 kPa) and slid back and forth on a common nylon fabric by moving 200 mm in each cycle.Fig. 7 shows the morphology and corresponding contact angle of the fabrics after a series of abrasion treatments. From the SEM image, it was found that the fabric was worn out after 5000 cycles of friction, and the rough structure of the surface was damaged and some fibers became flat. While CA only decreased from 164.4° ± 2.1° to 157.6° ± 3.4° (Fig. 7(d)), it still maintained superhydrophobicity (Fig. 7(c)). This slight decrease might be due to the partial loss of the surface roughness and the removal of the PVDF/PDMS coating under the severe friction. The excellent mechanical wear resistance of the as-prepared fabrics might be enabled by PDMS, an elastomer which has not only good wear resistance but also a strong adhesive force to fibers after curing.44,45
We also evaluated the laundering durability of the superhydrophobic fabrics by a modified laundering procedure. Fig. 8 is a photo of the inside of the laundering machine and schematic of the washing container, in which the fabrics were washed at 40 °C in the presence of 10 stainless steel balls with 0.37 wt% of detergent added. One washing cycle is approximately equal to five cycles of commercial laundering.
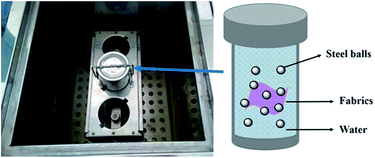 | ||
| Fig. 8 Photo of the inside of the laundering machine (left) and the illustration of the washing container. | ||
Fig. 9(a) and (b) shows the morphology of the fabrics after 30 cycles of washing which is approximately equal to 150 cycles of commercial laundering. It was found that the roughness of the coated fibers was partially removed. Fig. 9(d) shows the changes of the CA and SA with different washing times. The CA gradually decreased with the washing time, but was still greater than 150°. When the washing reached 150 times, the slight increase in CA was found, which may be due to the protruding fuzz introduced by the friction between the fibers and the balls under the washing process.41,45 It's worth emphasizing that the SA showed no great change with value less than 15°. It should be noted here too that the state of the surface was a transition state between the Cassie–Baxter and Wenzel model after a strong mechanical laundering. Although the roughness was partially lost, the fabric still maintained its superhydrophobicity. This indicates that the coated fabrics have excellent washing durability for superhydrophobicity. Fig. 9(c) shows the digital images of the water droplets that remained spherical both before and after 150 cycles of laundering.
Besides the excellent washing durability and abrasion resistance, the chemical resistance of the superhydrophobic fabrics was also evaluated. We immersed the superhydrophobic samples into different pH (pH = 1–13) solutions for 48 h, then rinsed them in water followed by drying. As shown in Fig. 10(a), the CAs still remained above 150°. This may be due to the air layer between the solution and the fibers preventing the fabrics from corrosion by acid or alkali solution, demonstrating that the fabric surface is highly stable against chemical corrosion.
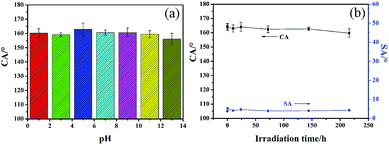 | ||
| Fig. 10 (a) Contact angle variation of different pH solutions after immersion for 48 h, (b) changes in CA and SA of the superhydrophobic fabrics after different UV light exposure times. | ||
In daily life, fabrics inevitably suffer from UV irradiation when exposed to sunlight. So the UV-resistant stability of the superhydrophobic fabrics was also evaluated. The superhydrophobic fabrics were placed under an ultraviolet light to determine their durability. Fig. 10(b) shows the CA and SA changes with different UV irradiation times. It was found that the CA only slightly decreased from 164.4° to 163.1° after 10 h of UV irradiation, and changed little after 150 h of irradiation. Importantly, the fabric remained superhydrophobic after the challenge of 9 days of UV irradiation with no great change in the value of SA. This indicates that the superhydrophobic fabrics have excellent resistant to the UV irradiation.
Self-cleaning of superhydrophobic fabrics
It is well known that fabrics are inevitably contaminated by dust, which brings a lot of inconvenience. The self-cleaning surface provided an effective way to solve this problem. For a superhydrophobic surface to be self-cleaning, a sliding angle lower than 10° is necessary. Therefore, we simulated an environment using dyes to detect the self-cleaning of the superhydrophobic fabrics. Fig. 11(a) shows the pristine fabric and the coated fabric with acid dyes on the samples. After dropping water on the dye, it can be seen that the pristine fabric was stained immediately due to their hydrophilicity, while the water droplet showed spherical and dyed color on the coated superhydrophobic fabric (Fig. 11(b)). After removing the water droplet, the coated fabric was still clean as before (Fig. 11(c)). This shows the excellent self-cleaning effect of the coated superhydrophobic fabrics.Conclusions
A robust superhydrophobic fabric with a PVDF/PDMS roughened coating was fabricated through a non-solvent induced phase-inversion method. The samples exhibited excellent wear resistance, laundering durability, acid and alkali resistance, UV irradiation and self-cleaning properties. In addition, this study offers a facile strategy to avoid the use of expensive equipment for fabricating robust superhydrophobic fabrics with coatings without adding extra nanoparticles. Therefore, the method is suitable for large-scale production.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51372146, 51572161), Program for New Century Excellent Talents in University (NCET-12-1042), Research Fund for the Doctoral Program of Higher Education of China (20136125110003), Major Program of Science Foundation of Shaanxi Province (2011ZKC05-7), Key Scientific Research Group of Shaanxi Province (2013KCT-08), and Scientific Research Group of Shaanxi University of Science and Technology (TD12-03).Notes and references
- Y. Lu, S. Sathasivam, J. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Science, 2015, 347, 1132–1135 CrossRef CAS PubMed.
- H. Chen, Z. Yuan, J. Zhang, Y. Liu, K. Li, D. Zhao, S. Li, P. Shi and J. Tang, J. Porous Mater., 2008, 16, 447–451 CrossRef.
- X. Deng, L. Mammen, H. J. Butt and D. Vollmer, Science, 2012, 335, 67–70 CrossRef CAS PubMed.
- X. Li, C. Zhou, R. Du, N. Li, X. Han, Y. Zhang, S. An and C. Xiao, ACS Appl. Mater. Interfaces, 2013, 5, 5430–5435 CAS.
- Z. Z. Luo, Z. Z. Zhang, L. T. Hu, W. M. Liu, Z. G. Guo, H. J. Zhang and W. J. Wang, Adv. Mater., 2008, 20, 970–974 CrossRef CAS.
- C. H. Xue, X. Q. Ji, J. Zhang, J. Z. Ma and S. T. Jia, Nanotechnology., 2015, 26, 335602–335610 CrossRef PubMed.
- C. H. Xue and J. Z. Ma, J. Mater. Chem. A, 2013, 1, 4146–4161 CAS.
- H. Yang, F. Liang, Y. Chen, Q. Wang, X. Qu and Z. Yang, NPG Asia Mater., 2015, 7, e176 CrossRef CAS.
- S. Peng, X. Yang, D. Tian and W. Deng, ACS Appl. Mater. Interfaces, 2014, 6, 15188–15197 CAS.
- C. Su, Y. Xu, F. Gong, F. Wang and C. Li, Soft Matter, 2010, 6, 6068–6071 RSC.
- L. Xu, Z. Geng, J. He and G. Zhou, ACS Appl. Mater. Interfaces, 2014, 6, 9029–9035 CAS.
- Q. Zhu, Y. Chu, Z. Wang, N. Chen, L. Lin, F. Liu and Q. Pan, J. Mater. Chem. A, 2013, 1, 5386–5393 CAS.
- S. Jung, M. Dorrestijn, D. Raps, A. Das, C. M. Megaridis and D. Poulikakos, Langmuir, 2011, 27, 3059–3066 CrossRef CAS PubMed.
- L. Mishchenko, B. Hatton, V. Bahadur, J. A. Taylor, T. Krupenkin and J. Aizenberg, ACS Nano, 2010, 4, 7699–7707 CrossRef CAS PubMed.
- Z. She, Q. Li, Z. Wang, L. Li, F. Chen and J. Zhou, Chem. Eng. J., 2013, 228, 415–424 CrossRef CAS.
- H. Wang, Z. Liu, E. Wang, R. Yuan, D. Gao, X. Zhang and Y. Zhu, Appl. Surf. Sci., 2015, 332, 518–524 CrossRef CAS.
- Y. Si, Q. Fu, X. Wang, J. Zhu, J. Yu, G. Sun and B. Ding, ACS Nano, 2015, 9, 3791–3799 CrossRef CAS PubMed.
- Y. Yu, H. Chen, Y. Liu, V. S. J. Craig, C. Wang, L. H. Li and Y. Chen, Adv. Mater. Interfaces, 2015, 2, 1400267–1400276 CrossRef.
- W. Zhang, Z. Shi, F. Zhang, X. Liu, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2071–2076 CrossRef CAS PubMed.
- M. P. Sousa and J. F. Mano, Cellulose, 2013, 20, 2185–2190 CrossRef CAS.
- C. Chen, J. Xu, Q. Zhang, Y. Ma, L. Zhou and M. Wang, Chem. Commun., 2011, 47, 1336–1338 RSC.
- Z. Sun, T. Liao, K. Liu, L. Jiang, J. H. Kim and S. X. Dou, Small, 2014, 10, 3001–3006 CrossRef CAS PubMed.
- M. He, Q. Zhang, X. Zeng, D. Cui, J. Chen, H. Li, J. Wang and Y. Song, Adv. Mater., 2013, 25, 2291–2295 CrossRef CAS PubMed.
- Y. Hou, Z. Wang, J. Guo, H. Shen, H. Zhang, N. Zhao, Y. Zhao, L. Chen, S. Liang, Y. Jin and J. Xu, J. Mater. Chem. A, 2015, 3, 23252–23260 CAS.
- Y. Li, S. Chen, M. Wu and J. Sun, Adv. Mater., 2014, 26, 3344–3348 CrossRef CAS PubMed.
- F. Su and K. Yao, ACS Appl. Mater. Interfaces, 2014, 6, 8762–8770 CAS.
- N. Wang, D. Xiong, Y. Deng, Y. Shi and K. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 6260–6272 CAS.
- Q. F. Xu, B. Mondal and A. M. Lyons, ACS Appl. Mater. Interfaces, 2011, 3, 3508–3514 CAS.
- S. Chen, X. Li, Y. Li and J. Sun, ACS Nano, 2015, 9, 4070–4076 CrossRef CAS PubMed.
- S. Barthwal, Y. S. Kim and S. H. Lim, Langmuir, 2013, 29, 11966–11974 CrossRef CAS PubMed.
- D. Ge, L. Yang, C. Wang, E. Lee, Y. Zhang and S. Yang, Chem. Commun., 2015, 51, 6149–6152 RSC.
- H. Wang, E. Wang, Z. Liu, D. Gao, R. Yuan, L. Sun and Y. Zhu, J. Mater. Chem. A, 2015, 3, 266–273 CAS.
- Y. Wang, Y. Shi, L. Pan, M. Yang, L. Peng, S. Zong, Y. Shi and G. Yu, Nano Lett., 2014, 14, 4803–4809 CrossRef CAS PubMed.
- C. H. Xue, X. J. Guo, J. Z. Ma and S. T. Jia, ACS Appl. Mater. Interfaces, 2015, 7, 8251–8259 CAS.
- Z. Chu and S. Seeger, RSC Adv., 2015, 5, 21999–22004 RSC.
- B. Wang, J. Li, G. Wang, W. Liang, Y. Zhang, L. Shi, Z. Guo and W. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 1827–1839 CAS.
- J. Zhang, B. Li, L. Wu and A. Wang, Chem. Commun., 2013, 49, 11509–11511 RSC.
- H. Zhou, H. Wang, H. Niu, J. Fang, Y. Zhao and T. Lin, Adv. Mater. Interfaces, 2015, 2, 1400559–1400566 CrossRef.
- X. Zhou, Z. Zhang, X. Xu, F. Guo, X. Zhu, X. Men and B. Ge, ACS Appl. Mater. Interfaces, 2013, 5, 7208–7214 CAS.
- M. Wu, B. Ma, T. Pan, S. Chen and J. Sun, Adv. Funct. Mater., 2016, 26, 569–576 CrossRef CAS.
- C. H. Xue, P. Zhang, J. Z. Ma, P. T. Ji, Y. R. Li and S. T. Jia, Chem. Commun., 2013, 49, 3588–3590 RSC.
- H. Zhou, H. Wang, H. Niu, A. Gestos and T. Lin, Adv. Funct. Mater., 2013, 23, 1664–1670 CrossRef CAS.
- C. H. Xue, X. J. Guo, M. M. Zhang, J. Z. Ma and S. T. Jia, J. Mater. Chem. A, 2015, 3, 21797–21804 CAS.
- H. Zhou, H. Wang, H. Niu, A. Gestos, X. Wang and T. Lin, Adv. Mater., 2012, 24, 2409–2412 CrossRef CAS PubMed.
- C. H. Xue, Y. R. Li, P. Zhang, J. Z. Ma and S. T. Jia, ACS Appl. Mater. Interfaces, 2014, 6, 10153–10161 CAS.
| This journal is © The Royal Society of Chemistry 2016 |