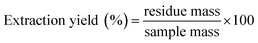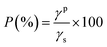Open Access Article
Open Access ArticlePhotoinitiated thiol–epoxy addition for the preparation of photoinduced self-healing fatty coatings
Yu Hui
Zhao
,
Daniela
Vuluga
,
Laurence
Lecamp
* and
Fabrice
Burel
Normandie Université, INSA de Rouen, CNRS UMR 6270, FR 3038, Avenue de l'Université, F-76801 Saint Etienne Du Rouvray Cedex, France. E-mail: laurence.lecamp@insa-rouen.fr
First published on 24th March 2016
Abstract
Herein, we report the use of the photoinitiated thiol–epoxy chemistry for the preparation of a new biosourced self-healing coating. Epoxidized cottonseed oil is used as the main reagent (>80 wt%) and photocrosslinked in the presence of 0.25 equivalents of 7-mercapto-4-methyl coumarin, used as a photodimerizable and healable reactant. The reaction is initiated with 2 wt% of a photobase generator (PBG). The stability of the thiol–epoxy mixture under storage and the oxygen-promoted photoinitiating system are some of the advantages illustrated in this study. The physicochemical properties of the resulting coatings as well as the photoreversibility of the coumarin derivative once grafted on the vegetable oil are studied. The photoinitiation step is demonstrated to be efficient for the grafting of the healing reagent as well as for epoxy homopolymerization.
Introduction
Nowadays, click chemistry involving thiol molecules is widely used for the functionalization of molecules bearing carbon–carbon double bonds, the preparation of polymers, or the crosslinking of polymer materials. Although this chemistry can be applied on all carbon–carbon unsaturation types, this particular type usually requires high thiol amounts compared to ene molecules, long reaction times and leads to poor yield when applied to fatty double bonds, due to their low reactivity and numerous side reactions.1–4 On the other side, addition of thiol on epoxy groups can be an interesting alternative pathway. Thus, the thiol–epoxy reaction is generally used to prepare adhesives or plastic hardeners at industrial scale by thermal methods.5 Recently, this reaction has attracted lots of attention in polymer chemistry as both hydroxy and thioether groups can be formed at once and even be further transformed.6,7 This reaction may be initiated by a tertiary amine and has been already used in few works to successfully prepare polymer materials.8–10 Surprisingly, the thiol–epoxy reaction under UV was scarcely studied until now. Literature only reports the recent development of convenient photobase generators (PBG) for such a reaction.9,11–16 Hence, to the best of our knowledge, this photoinitiated reaction has never been used until now to prepare polymer materials.The elaboration of photo-healable materials has already been the subject of several works. The photo-healing property is mostly brought by photosensitive molecules or functional groups such as coumarine,17,18 anthracene,19 trithiocarbonate units,….20 These photosensitive moieties are grafted on synthetic or natural macromolecular chains,17,19 or introduced in a (co-)monomer before its incorporation in a polymer network.18,20 The synthesis process of such materials therefore requires at least two steps.
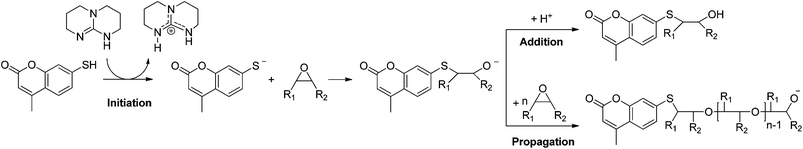
This paper proposes a one-step process to prepare a photo-healable coating from epoxidized vegetable oil by using the photoinitiated thiol–epoxy addition. Epoxidized cottonseed oil (ECO) was chosen as raw material and 7-mercapto-4-methyl-coumarin (MMC) was selected as photo-healing reagent. Indeed, the coumarin molecule is well known for its reversible dimerization under UV irradiation.21–24 The selected coumarin derivative could be grafted onto epoxidized triglycerides thanks to its SH group. Hence, the thiol–epoxy addition and the dimerization of coumarin groups are expected concomitant under UV irradiation to produce an original photocrosslinked and photo-healable fatty coating (Scheme 1).
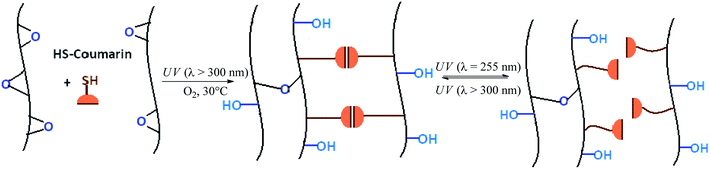 | ||
| Scheme 1 Coating preparation by photoinitiated thiol–epoxy reaction between epoxidized cottonseed oil and 7-mercapto-4-methyl coumarin under UV and air and its photoinduced self-healing behavior. | ||
This work is presented in two parts. In the first part, the influence of oxygen, photoinitiating system amount and thiol/epoxy ratio on the photoinitiated thiol–epoxy reaction kinetics will be studied. The second part will consist in the characterization of the coating prepared according to the determined optimal conditions in terms of physico-chemical properties as well as evaluation of photo-healing ability.
Experimental
Materials
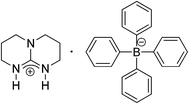
Epoxidized cottonseed oil was kindly prepared by the Laboratoire de Sécurité des Risques Chimiques (LSPC, INSA de Rouen, France).25 7-Mercapto-4-methyl-coumarin (MMC, 97%) purchased from Sigma Aldrich, and 12-dodecanethiol (98%) obtained from Merck-Schuchardt were used as thiol reactants. Deuterated chloroform, dichloromethane (>99%) and acetone (>99%) were supplied by VWR. All materials were used as received.The photobase generator (PBG), presented in Scheme 2, was prepared as described in the literature.26 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD, 98%) and sodium tetraphenylborate (NaBPh4, 100%) were purchased from Sigma Aldrich. Chlorohydric acid (37%) was bought from Acros. Isopropylthioxanthone (ITX, >99%) was kindly supplied by IGM resins and 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO, 98%) was purchased from Acros.
Kinetic study of the photochemical reactions
Formulations with molar dodecanethiol/epoxy ratios ranging from 0.25 to 1 were prepared in acetone at a 900 μg mL−1 concentration. Formulations with molar MMC/epoxy ratios equal to 0.25 and 0.5 were prepared in dichloromethane at the same 900 μg mL−1 concentration. A three-component (0.5 wt% ITX + 1 wt% TBD·HBPh4 + 0.5 wt% TEMPO) photoinitiating system was added to each formulation. Reagents were stirred during 1 min.Photo-reversibility study of MMC
A 900 mg mL−1 solution of MMC in dichloromethane was casted on a quartz plate. The dimerization process was carried out by placing the plate at 10 cm from a polychromatic light coming from a Hg–Xe lamp (Hamamatsu LC8) fitted with a filter selecting wavelengths between 300 nm and 450 nm. The incident light intensity was measured at 365 nm and set to 120 mW cm−2. For the retro-reaction, a 254 nm interferential filter was used and the intensity of the monochromatic light was kept at 0.6 mW cm−2.Coatings preparation
A 0.25 molar thiol/epoxy ratio and 2 wt% PBG were used. Formulation was prepared at a 90 μg mL−1 concentration in dichloromethane, stirred during 1 min and then applied onto a glass substrate. The film was then irradiated under air for 20 min by a filtered polychromatic light coming from a Hg–Xe lamp (Hamamatsu LC8). The emission spectrum was restricted to the range 300–450 nm and the incident light intensity measured at 365 nm was 120 mW cm−2.Analytical techniques
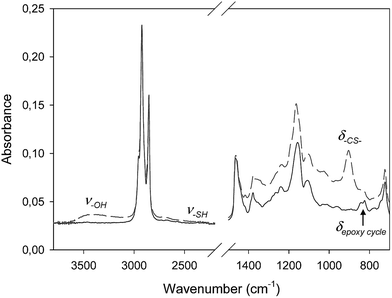
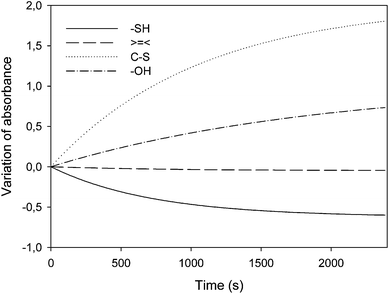
1H NMR analyses were carried out on a Bruker 300 MHz spectrometer at room temperature. The chemical shifts are given in ppm relative to trimethylsilane (TMS) used as external reference. Samples were prepared as follow: 10 mg of product in 0.4 mL of CDCl3.Fourier Transform Infra-Red (FTIR) spectra were performed on a Vertex 70 Bruker spectrometer equipped with an ATR accessory (ATR MK II Golden Gate Specac, Eurolabo) at 30 °C. A drop of the sample was applied to the horizontal ATR crystal and then covered or not by a polyethylene film before irradiation. The UV light coming from a polychromatic Hg–Xe lamp (Hamamatsu LC8) was applied while IR spectra were recorded versus time. Epoxy group, thiol function and coumarin's double bond consumption was determined by following the absorbance of the peaks at 823 cm−1 (deformation band of oxirane cycle), 2550 cm−1 (S–H stretching band) and 3100 cm−1 (symmetric stretching band of cis configuration alkene), respectively. The formation of thioether bond and hydroxyl group was monitored by following the absorbance of the peaks at 900 cm−1 (C–S deformation band) and 3500 cm−1 (OH stretching band), respectively. Reaction kinetics was plotted using the absorbance variation of the different peaks: ΔA = A0 − At where A0 and At refer to the absorbance of the corresponding peak at t = 0 and t, respectively.
UV spectra were performed using a Cary 100 Varian UV-visible spectrometer. The formulation was spread out on a quartz window and analyzed between 200 and 400 nm.
Size Exclusion Chromatography (SEC) analyses were carried out with a Varian 50 system and equipped with a pre-column (Polymer Laboratories, PL gel 5 μm Guard, 50 × 7.5 mm) and two columns (Polymer Laboratories, 2 PL gel 5 μm MIXED-D columns, 2 × 300 × 7.5 mm) and a refractive index detector (differential refractometer, sensibility: 2.5 × 107 RIU per mV, wavelength 880 ± 30 nm). Dichloromethane was used as eluent with a 1 mL min−1 flow at 35 °C. The system was calibrated using polymethylmethacrylate (PMMA) standards. Samples were analysed with an internal standard (PMMA, 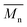 = 29
= 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1, 20% of the sample mass) after filtration (0.45 μm).
600 g mol−1, 20% of the sample mass) after filtration (0.45 μm).
Thermogravimetric analyses (TGA) were performed on a TGA Q500 device from TA Instruments (platinum pan, 10 mL min−1 constant flow of nitrogen, temperature range from 30 °C to 600 °C, 10 °C min−1 heating rate).
Differential Scanning Calorimetry (DSC) analyses were performed on a DSC Q2000 device from TA Instruments (aluminum pan, 10 mL min−1 constant flow of nitrogen, temperature range from −80 °C to 120 °C, 10 °C min−1 heating rate).
Film extraction was carried out as follows: 50 mg of sample were added into a 100 mL flask and extracted with 70 mL dichloromethane. The extraction was carried out for 24 h in cellulose vessels, under atmospheric pressure and at the temperature of 60 °C (reflux). The extraction yield was determined from weighing of the residue:
The extraction liquids were collected, concentrated in a rotary evaporator at 40 °C and then analyzed by SEC according to the aforementioned protocol.
Surface energy of the materials was evaluated using a Digidrop Goniometer (GBX, France). Static contact angles were measured at the equilibrium time with three liquids: water, glycerol and diiodomethane. Five measurements were performed for each sample surface to calculate the average contact angle and its standard deviation. According to the Owens–Wendt relationship, the dispersive γd and polar γp components and the surface energies (γs = γd + γp) of the samples were determined. Surface polarities (P) of the materials were also calculated using equation:
Film hardness was investigated using a König pendulum SP0500 (Labomat Essor). Three measurements were performed for each sample to calculate the average König hardness and its standard deviation.
Results and discussion
Thiol–ene addition is the usual pathway for the functionalization of unsaturated vegetable oils by thiol reagents.27,28 Nevertheless, this pathway presents many disadvantages such as instability of the reaction medium under storage and numerous side reactions.29 For these reasons, the thiol–epoxy addition was preferred to the thiol–ene one in this work. The fatty raw material was a cottonseed oil having 4 ethylenic double bonds per triglyceride molecule in its native form (determined by 1H NMR analysis).30 A peroxidative process was used as mentioned in the experimental part to prepare epoxidized oil. 1H NMR analysis of the ECO showed an average number of 4 epoxy groups per triglyceride molecule (the presence of residual ethylenic double bond was estimated to 7%). 7-Mercapto-4-methyl-coumarin (MMC) was chosen as thiol monomer and for its photo-reversible cyclization.According to literature, thiol–epoxy addition can be initiated by tertiary amines. Two initiation pathways can occur as a function of the pKa of initiator and thiol group.31 In the presence of a good hydrogen donor (e.g. thiol with low pKa), base initiation is observed. In this case, the base can abstract the thiol hydrogen to form a thiolate anion, which then opens the oxyrane group. In the opposite case, a nucleophilic initiation rather occurs. Thus, an alcoolate group is formed by addition of the amine onto the epoxy function before addition on the thiol function. An anionic epoxy homopolymerization can then ensue when there is no more hydrogen donor in the reaction medium.14
In this work, the photochemical initiation way was privileged. For that, a photobase generator composed of a latent triamine (TBD·HBPh4) and a photosensitizer (ITX) in 1/0.5 wt% proportions, respectively, was used. As the basicity of the PGB is very strong (pKa = 15.2)26 and far greater than the acidity of the thiol coumarin derivative (pKa = 7–8),17 the initiation step can be considered as a base pathway (Scheme 3). It was also noticed that 0.5 wt% of TEMPO was added as radical inhibitor.
Model kinetic study of the thiol–epoxy reaction
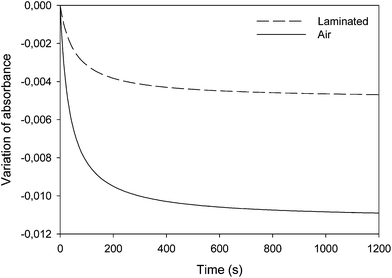
In order to determine the optimal composition of the thiol/oil formulation, a kinetic study of the thiol/epoxy reaction was first carried out. To model the MMC behavior, dodecanethiol (DT, pKa = 10.2)17 was chosen due to its good solubility into oil. Reaction kinetics was monitored by real time infrared spectroscopy at 30 °C. A polychromatic UV light with an incident intensity of 120 mW cm−2 (measured at 365 nm) was used. Although absorption bands corresponding to S–H bond at 2550 cm−1 and oxirane group at 823 cm−1 can be observed on IR spectra, the low intensity of the first band and the poor resolution of the second one did not allow calculating conversions accurately as shown on Fig. 1. Hence, only the epoxy absorbance decrease kinetics was presented here. Besides, the absorbance increase of C–S bond and OH group was also followed.The influence of oxygen onto thiol–epoxy addition was firstly investigated. A stoichiometric thiol/epoxy formulation was prepared and spread out on the ATR crystal. Samples were then irradiated under air or in laminated layer in the presence of 2 wt% of PBG. Fig. 2 shows that the absorbance variation kinetics for the epoxy group was greater under air. Hu et al. have recently shown that the UV exposure of aromatic amines similar to TBD in the presence of photosensitizer could induce numerous catalyzed redox reactions.15 The greater PBG's reactivity observed under air and UV radiation could possibly be explained by such reactions that could induce a faster base releasing. The following experiments were then carried out under air.
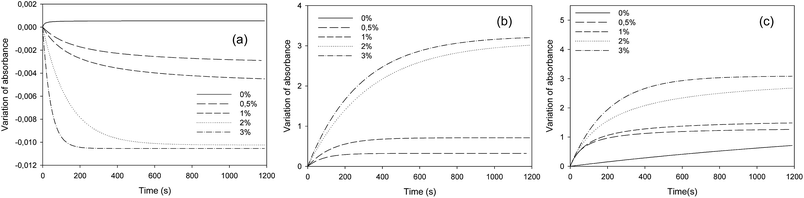
The PBG amount was then varied from 0 to 3 wt%. As expected, the results showed that the consumption of epoxy functions (Fig. 3a) as well as the formation of C–S bond and OH group (Fig. 3b and c, respectively) was more important as the quantity of PBG increased. Nonetheless, this trend seems to diminish for higher PGB concentrations, probably due to the limited solubility of PBG into oil. We therefore decided to keep a PBG concentration of 2 wt% in the following experiments. It is also noteworthy that no C–S bond appeared in the absence of PBG, indicating the requirement of the photocatalytic system to initiate the reaction confirming the stability of the mixture under storage. However, we also observed a slight formation of epoxy and OH groups in this case. This could be explained by the presence of hydroperoxydes in the vegetable oil, which are well known to generate 1O2 under UV radiation.32,33 This oxygen molecule could then oxidize the residual ethylenic double bonds of the epoxidized cottonseed oil.
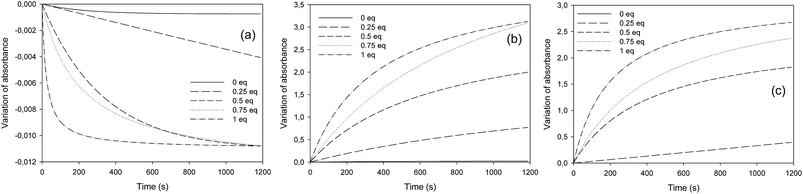
Last, the influence of thiol quantity (from 0 to 1 equivalent SH relative to epoxy function) was studied. As expected, Fig. 4 shows that the epoxy consumption (Fig. 4a) as well as the C–S bond and OH bond formation (Fig. 4b and c, respectively) progressed more quickly when the quantity of thiol was more important. The soluble fractions of the formulations before and after irradiation were analyzed by SEC with an internal standard after filtration. Results showed that higher concentrations of thiol increased the dimerization of thiol monomers (11% for 0.5 eq. MMC against 6% for 0.25 eq. MMC). As a result, thiol–epoxy addition was much lower. We therefore decided to use a molar thiol/epoxy ratio of 0.25 in the following study.
Kinetic study of the MMC photo-reversibility
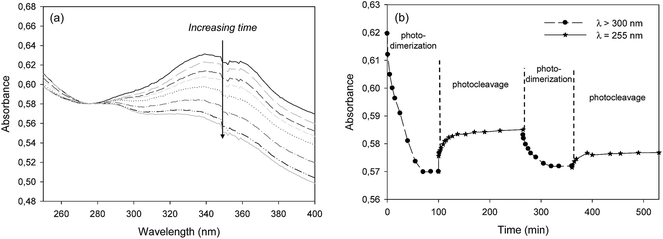
The photo-reversibility of the (non grafted) MMC at solid state was firstly studied. MMC was first solubilized in dichloromethane and spread out on a quartz plate. After solvent evaporation, MMC was first irradiated by a polychromatic light (300 < λ < 450 nm – I0 = 120 mW cm−2 at 365 nm) under air for 90 min and then by a monochromatic light (λ = 255 nm – I0 = 0.6 mW cm−2) under air for 120 min. The photo-reversibility of the coumarin moiety was monitored by UV-visible spectrophotometry (Fig. 5). Indeed, the coumarin group initially exhibited an absorbance maximum at λmax = 330–340 nm.34 When exposed to the UV-B/C radiation, the [2 + 2] cycloaddition occurred and led to a decrease of this absorbance maximum until a plateau was reached after 60 min irradiation (Fig. 5a). By exposure to the 255 nm radiation, an absorbance increase was observed (Fig. 5b), indicating the photocleavage of the cyclobutane and the reformation of double bonds (Scheme 4a). In the course of the second irradiation cycle, the photo-reversibility of MMC was again observed. However, we could remark the absorbance variation amplitude was lower than after the first irradiation cycle (Fig. 5b). | ||
| Scheme 4 Possible MMC's dimerization pathways under UV radiation: (a) [2 + 2] cycloaddition (major); (b) thiol–ene addition (minor). | ||
A further kinetic monitoring of the photochemical reaction by FTIR spectroscopy well highlighted not only the variation of the double bonds band absorbance during the different irradiation steps, but also the appearance of the C–S absorption band at 900 cm−1. The formation of C–S bond could only be explained by a thiol–ene reaction (Scheme 4b) between the thiol group of MMC and its double bond via a radical process, which is favoured by the high MMC concentration. This reaction could probably explain the attenuation of the photo-reversibility after several irradiation cycles.
Once grafted onto the epoxidized cottonseed oil and the formulation photocured, the photo-reversibility of the MMC was secondly studied at solid state. Formulation composed of a 0.25 molar thiol/epoxy ratio and 2 wt% PBG was spread out on quartz plate and exposed to similar irradiation conditions as previously described. Fig. 6 showed that the mixture before irradiation was opaque and non homogeneous whereas the coating was entirely transparent and homogeneous after irradiation, meaning a good incorporation of MMC by grafting onto oil. It is noteworthy that the reaction was carried out in the presence of TEMPO. Hence, the MMC grafting via the thiol–epoxy reaction rather occurred than the radical thiol–ene addition.
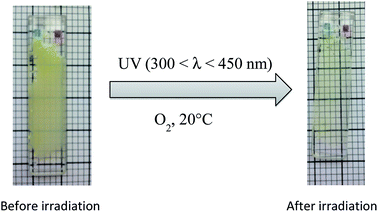 | ||
Fig. 6 Films composed of cottonseed oil + dodecanethiol (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 in functions) + 2 wt% PBG before and after irradiation at 300 < λ < 450 nm (I0 = 120 mW cm−2 at 365 nm). 0.25 in functions) + 2 wt% PBG before and after irradiation at 300 < λ < 450 nm (I0 = 120 mW cm−2 at 365 nm). | ||
As in the previous study, the photo-reversibility of the grafted MMC was monitored by FTIR and UV-visible spectroscopies. Fig. 7 presents the kinetics of MMC's double bond and SH function consumption and of C–S bond and –OH group formation.
Characterization of materials
10–20 μm thick films were elaborated according to the optimal conditions previously determined. A formulation containing 0.25 eq. MMC relative to fatty epoxy group and 2 wt% of PGB was then irradiated at 30 °C with a polychromatic light from 300 to 450 nm and an incident light intensity I0 = 120 mW cm−2 measured at 365 nm. The physicochemical properties of the films were then characterized.In a general way, the photocured films are transparent and flexible. They do not release any thiol smell and are not sticky after irradiation. FTIR analyses of the photocured films showed a complete consumption of both MMC's double bond and SH group as well as the consumption of epoxy functions and the formation of OH groups. Furthermore, extraction was carried out on the obtained films and extractables were analyzed by both 1H NMR and SEC in the presence of an internal standard. The soluble fraction was estimated to 65% and was mainly composed of 80 wt% of epoxidized triglycerides (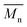 = 990 g mol−1 – Đ = 1.03) and 16 wt% of a mixture of thiol–epoxy adducts and/or triglyceride oligomers with low molecular weight (
= 990 g mol−1 – Đ = 1.03) and 16 wt% of a mixture of thiol–epoxy adducts and/or triglyceride oligomers with low molecular weight (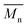 = 2700–3200 g mol−1 – Đ = 1.08). Only 2 wt% of MMC and 2 wt% of MMC dimers were detected by SEC whereas NMR 1H analysis showed no residual thiol after irradiation. All these results showed that MMC was well grafted onto epoxidized triglycerides and dimerized.
= 2700–3200 g mol−1 – Đ = 1.08). Only 2 wt% of MMC and 2 wt% of MMC dimers were detected by SEC whereas NMR 1H analysis showed no residual thiol after irradiation. All these results showed that MMC was well grafted onto epoxidized triglycerides and dimerized.
TGA and DSC analyses of the photocured films before and after extraction as well as of the epoxidized cottonseed oil were performed. Fig. 8 highlighted that the films' degradation could be divided into two parts, which were more pronounced for the washed film. The first part could be attributed to the degradation of free triglycerides and/or its oligomers till 300 °C,35 whereas the second one could be related to the degradation of aromatic structures. Furthermore, it could be noticed that the degradation temperature of the films was lower than the epoxidized cottonseed oil one. This could be explained by the thermally activated cleavage of C–S or S–S bond, which exhibit lower bond energy than C–C and C–O ones.36 Besides, according to DSC analysis, the film after extraction exhibited a glass transition temperature of −13 °C. This temperature was higher than the one measured before extraction, free low molecular weight molecules acting as plasticizer. Despite of this low Tg value, the non washed film exhibited a relatively high König hardness estimated to 102 ± 18 s. Last, the polarity of the photocured film was estimated to 15 ± 4%.
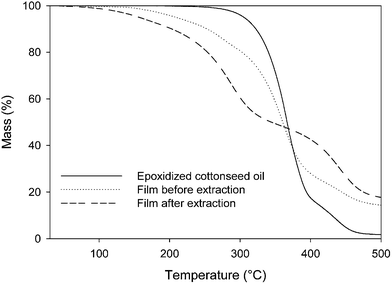 | ||
| Fig. 8 Thermal degradation curves for epoxidized cottonseed oil and photocured films before and after extraction. | ||
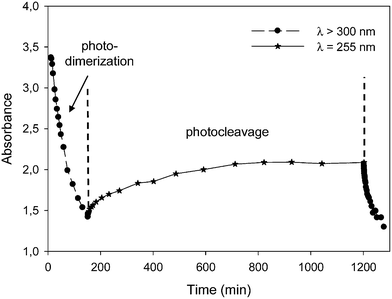
To finish, the photo-reversibility properties of the film were evaluated. Several irradiation cycles were applied. Each cycle was consisted of 180 min irradiation at 300 < λ < 450 nm (I0 = 120 mW cm−2 at 365 nm) and 8 h irradiation at λ = 255 nm (I0 = 0.6 mW cm−2). The monitoring of the MMC photo-reversibility by UV spectroscopy (Fig. 9) showed a decrease of the absorbance maximum at 330 nm from 3.4 to 1.4 during the irradiation step at 300 < λ < 450 nm, indicating the MMC dimerization. The photodimerized film was then subjected to the radiation at λ = 255 nm. With the progress of the retro-dimerization, the film absorbance at 330 nm slowly increased from 1.4 to 2.0 after 8 h irradiation. This great reaction time and the limited recovery could be explained by the very weak irradiation intensity, which was delivered by the Hg–Xe lamp at 255 nm. A new irradiation at 300 < λ < 450 nm led again to a decrease of the maximum absorbance of the MMC consecutive to its dimerization. The photo-reversibility of the film was therefore confirmed.
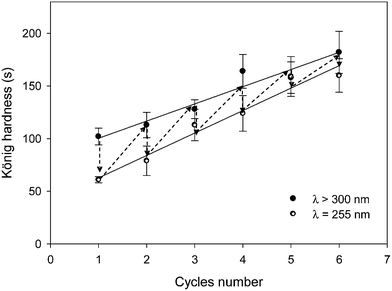
The self-healability of the system was also quantified by pendulum hardness measurement. The König hardness of the film was determined after each irradiation step and reported in Fig. 10 as a function of the irradiation cycles number. Results highlighted several interesting points. First, the decrease of the film hardness after irradiation at λ = 255 nm could be considered as the best evidence for the photocleavage of the grafted MMC dimer into the grafted MMC monomer. Second, hardness can be recovered after irradiation at 300 < λ < 450 nm, confirming again the photo-reversibility of the dimerization. However, it is noteworthy that the restored hardness was higher than the one measured in the previous irradiation cycle. The significant increase of the film hardness in the course of the irradiation steps could be explained by the homopolymerization of epoxy groups that occurred and/or continued under all irradiation conditions, as confirmed by FTIR. The resulting higher crosslinking density could also induce a reducing of the coumarin moieties mobility and explain the limitation of the reversibility recovery as observed in Fig. 9.
Conclusions
In this study, a novel biosourced photo-healable material was prepared by photoinitiated thiol–epoxy chemistry. This new coating is constituted of 80 wt% of epoxidized cottonseed oil and 20 wt% of 7-mercapto-4-methyl coumarin. The reaction could be performed under ambient conditions (room temperature and air). The MMC's photografting onto epoxidized vegetable oil via the thiol–epoxy addition and its photodimerization were clearly confirmed. Furthermore, the ring opening polymerization of fatty epoxy groups was also observed in the presence of PBG. This side reaction enhanced the crosslinking density of the final coating in the course of the photo-healing steps. At last, it could be noteworthy that this is the first photoinduced self-healing material prepared by photoinitiated thiol–epoxy chemistry from vegetable oil derivatives.Acknowledgements
This work has been supported by the French Government (MESR fellowship). The authors are grateful to S. Leveneur and J.-L. Zheng of the Laboratoire de Sécurité des Risques Chimiques (LSPC, INSA de Rouen, France) for the epoxidized cottonseed oil preparation.Notes and references
- O. Türünç and M. A. R. Meier, Green Chem., 2011, 13, 314 RSC.
- M. Desroches, S. Caillol, V. Lapinte, R. Auvergne and B. Boutevin, Macromolecules, 2011, 44, 2489 CrossRef CAS.
- A. Corneille, V. Froidevaux, C. Negrell, S. Caillol and B. Boutevin, Polymer, 2014, 55, 5561 CrossRef.
- C. Lluch, J. C. Ronda, M. Galia, G. Lligadas and V. Cadiz, Biomacromolecules, 2010, 11, 1646 CrossRef CAS PubMed.
- G. L. Schneberger, Adhesives in Manufacturing, Marcel Dekker Inc, 1983, vol. 11 Search PubMed.
- A. Brändle and A. Khan, Polym. Chem., 2012, 3, 3224 RSC.
- S. Binder, I. Gadwal, A. Bielmann and A. Khan, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2040 CrossRef CAS.
- K. Arimitsu and R. Endo, Chem. Mater., 2013, 25, 4461 CrossRef CAS.
- C. F. Carlberg, A. Vastesson, Y. Liu, W. van der Wijingaart, M. Johansson and T. Haraldsson, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2604 CrossRef.
- J. A. Carioscia, J. W. Stansbury and C. N. Bowman, Polymer, 2007, 48, 1526 CrossRef CAS PubMed.
- S. Katogi and M. Yusa, J. Polym. Sci., Part A: Polym. Chem., 2011, 40, 4045 CrossRef.
- C. M. Seubert and M. E. Nicols, J. Coat. Technol. Res., 2010, 7, 615 CrossRef CAS.
- D. P. Nair, M. Podgorski, S. Chantani, T. Gong, W. Xi, C. R. Fenoli and C. N. Bowman, Chem. Mater., 2014, 26, 724 CrossRef CAS.
- J. Lalevée, M. A. Tehfe, F. Morlet-Savary, B. Graff, X. Allonas and J. P. Fouassier, Prog. Org. Coat., 2011, 70, 23 CrossRef.
- J. Hu, J. Wang, T. H. Nguyen and N. Zheng, Beilstein J. Org. Chem., 2013, 9, 1977 CrossRef PubMed.
- J. A. Carioscia, J. W. Stansbury and C. N. Bowman, Polymer, 2007, 48, 1526 CrossRef CAS PubMed.
- S. Banerjee, R. Tripathy, D. Cozzens, T. Nagy, S. Keki, M. Zsuga and R. Faust, ACS Appl. Mater. Interfaces, 2015, 7, 2064 CAS.
- B. Kiskan and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2911 CrossRef CAS.
- P. Froimowicz, H. Frey and K. Landfester, Macromol. Rapid Commun., 2011, 32, 468 CrossRef CAS PubMed.
- Y. Amamoto, J. Kamada, H. Otsuka, A. Takahara and K. Matyjaszewski, Angew. Chem., Int. Ed., 2011, 50, 1660 CrossRef CAS PubMed.
- M. Nagata and Y. Yamamoto, React. Funct. Polym., 2008, 68, 915 CrossRef CAS.
- Y. Chujo, K. Sada and T. Saegusa, Macromolecules, 1990, 23, 2693 CrossRef CAS.
- J. Ling, M. Z. Rong and M. Q. Zhang, Polymer, 2012, 53, 2691 CrossRef CAS.
- E. Sato, S. Nagai and A. Matsumoto, Prog. Org. Coat., 2013, 76, 1747 CrossRef CAS.
- J. L. Zheng, J. Wärna, F. Burel, T. Salmi, B. Taouk and S. Leveneur, AIChE J., 2016, 62, 726 CrossRef CAS.
- X. Sun, J. P. Gao and Z. Y. Wang, J. Am. Chem. Soc., 2008, 130, 8130 CrossRef CAS PubMed.
- M. Desroches, S. Caillol, V. Lapinte, R. Auvergne and B. Boutevin, Macromolecules, 2011, 44, 2489 CrossRef CAS.
- M. Ionescu, D. Radojcic, X. Wan, Z. S. Petrovic and T. A. Upshaw, Eur. Polym. J., 2015, 67, 439 CrossRef CAS.
- C. N. Bowman and C. E. Hoyle, Angew. Chem., Int. Ed., 2010, 49, 1540 CrossRef PubMed.
- O. Zovi, L. Lecamp, C. Loutelier-Bourhis, C. M. Lange and C. Bunel, Eur. J. Lipid Sci. Technol., 2011, 113, 616 CrossRef CAS.
- K. Dietliker, R. Hüsler, J.-L. Birbaum, S. Ilg, S. Villeneuve, K. Studer, T. Jung, J. Benkhoff, H. Kura, A. Matsumoto and H. Oka, Prog. Org. Coat., 2007, 58, 146 CrossRef CAS.
- J. Regensburger, T. Maisch, A. Knak, A. Gollmer, A. Felgenträger, K. Lehner and W. Bäumler, Phys. Chem. Chem. Phys., 2013, 15, 17672 RSC.
- J. Regensburger, A. Knak, T. Maisch, L. Landthaler and W. Bäumler, Exp. Dermatol., 2011, 21, 135 CrossRef PubMed.
- G. Kaur, P. Johnston and K. Saito, Polym. Chem., 2014, 5, 2171 RSC.
- R. Wang and T. P. Schuman, eXPRESS Polym. Lett., 2013, 7, 272 CrossRef CAS.
- S. W. Benson, Chem. Educ. Eur., 1965, 42, 502 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |