Discrimination of enantiomers of dipeptide derivatives with two chiral centers by tetraaza macrocyclic chiral solvating agents using 1H NMR spectroscopy†
Lixia
Fang
a,
Caixia
Lv
a,
Guo
Wang
b,
Lei
Feng
a,
Pericles
Stavropoulos
c,
Guangpeng
Gao
a,
Lin
Ai
*a and
Jiaxin
Zhang
*a
aCollege of Chemistry, Beijing Normal University, Beijing 100875, P. R. China. E-mail: linai@bnu.edu.cn; jxzhang@bnu.edu.cn
bDepartment of Chemistry, Capital Normal University, Beijing 10048, P. R. China
cDepartment of Chemistry, Missouri University of Science and Technology, Rolla, Missouri 65409, USA
First published on 30th September 2016
Abstract
1H NMR spectroscopy is often used to discriminate enantiomers of chiral analytes and determine their enantiomeric excess (ee) by various chiral auxiliaries. In reported research, these studies were mainly focused on chiral discrimination of chiral analytes with only one chiral center. However, many chiral compounds possessing two or more chiral centers are often found in natural products, chiral drugs, products of asymmetric synthesis and biological systems. Therefore, it is necessary to investigate their chiral discrimination by effective chiral auxiliaries using 1H NMR spectroscopy. In this paper, a new class of tetraaza macrocyclic chiral solvating agents (TAMCSAs) with two amide (CONH), two amino (NH) and two phenolic hydroxyl (PhOH) groups has been designed and synthesized for chiral discrimination towards dipeptide derivatives with two chiral centers. These dipeptide derivatives are important chiral species because some of them are used as clinical drugs and special dietary supplements for treatment of human diseases, such as L-alanyl-L-glutamine and aspartame. The results show that these TAMCSAs have excellent chiral discriminating properties and offer multiple detection possibilities pertaining to 1H NMR signals of diagnostic split protons. The nonequivalent chemical shifts (up to 0.486 ppm) of various types of protons of these dipeptide derivatives were evaluated with the assistance of well-resolved 1H NMR signals in most cases. In addition, enantiomeric excesses (ee) of the dipeptide derivatives with different optical compositions have been calculated based on integration of well-separated proton signals. At the same time, the possible chiral discriminating behaviors have been discussed by means of Job plots, ESI mass spectra and a proposed theoretical model of (±)-G1 with TAMCSA 1c. Additionally, the association constants of enantiomers of (±)-G5 with TAMCSA 1a were calculated by employing the nonlinear curve-fitting method.
Introduction
In the field of chiral molecular recognition, NMR spectroscopy has been extensively utilized as one of the most effective techniques towards discriminating enantiomers of various chiral analytes by virtue of chiral auxiliaries,1 such as chiral derivatizing agents (CDAs),2 chiral lanthanide shift reagents (CLSR),3 chiral shift reagents (CSRs)4 and chiral solvating agents (CSAs).5 Compared with chiral chromatography and X-ray crystallography, the more prominent advantages of NMR spectroscopy are its attributes as a fast, accurate and convenient method.6 In reported papers, NMR spectroscopy was mainly focused on enantiodifferentiation of chiral analytes with only one chiral center by chiral auxiliaries.7 However, studies on enantiomeric discrimination of chiral compounds with two or more chiral centers were rarely reported in the past.8 Nevertheless, these chiral compounds often exist in natural products, chiral drugs, products of asymmetric synthesis and biological systems.9 Therefore, it is necessary to intensively develop more effective chiral auxiliaries for their chiral discrimination by 1H NMR spectroscopy.10 Herein, dipeptide derivatives were selected as one of the most representative species with two stereo configurations. Generally, dipeptides or their derivatives contain two identical or different chiral amino acid residues with one or two stereocenters. Thus, these dipeptides or their derivatives are likely composed of one, two, three and even four stereoisomers (D,D; L,L; D,L; L,D) under the same amino acid sequence, due to the reasons noted above. Of course, they are also achiral compounds. However, different stereoisomers have different biological and pharmacological activities, and even result in the opposite physiological effect.11 Therefore, analysis and determination of the optical compositions of these dipeptides or their derivatives, especially used as clinical drugs and special dietary supplements (such as L-alanyl-L-glutamine12 and aspartame13), are of important significance for the treatment of human diseases. Furthermore, this work addresses a challenging and fundamental problem, due to the very similar chemical structures and more complicated stereochemistries involved. In this paper, we report methodology towards achieving highly effective chiral discrimination of dipeptide derivatives with two chiral centers, in the presence of a new class of tetraaza macrocyclic chiral solvating agents (TAMCSAs) 1a–1c, by employing 1H NMR spectroscopy.Results and discussion
Design and synthesis of TAMCSAs 1a–1c for chiral discrimination
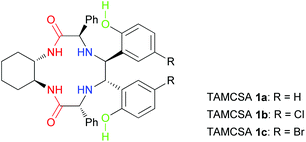
TAMCSAs 1a–1c have been designed and synthesized from D-(−)-α-phenylglycine and (1S,2S)-(+)-1,2-diaminocyclohexane for chiral discrimination of dipeptide derivatives with two chiral centers by 1H NMR spectroscopy.14 The detailed procedure of synthesis of TAMCSAs 1a–1c is available in the ESI.† Their chemical structures are shown in Fig. 1.As shown in Fig. 1, the most outstanding features of TAMCSAs 1a–1c are the presence of two amide, two amino and two phenolic hydroxyl groups as potential intermolecular interaction sites. Other notable features include an overall C2-symmetry and a 12-membered cavity as part of the framework.
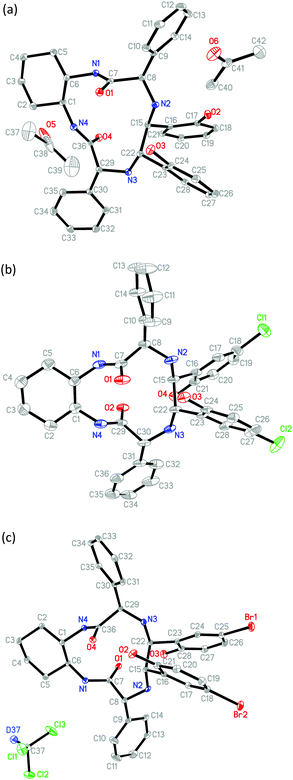
The newly generated chiral centers of TAMCSAs 1a–1c in the reductive coupling reaction are assigned to possess S, S absolute configurations based on their X-ray crystallographic analysis. Their X-ray crystal structures are shown in Fig. 2.
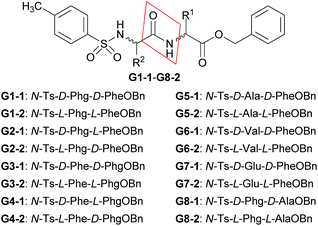
To investigate the chiral discriminating ability of TAMCSAs 1a–1c towards dipeptide derivatives with two chiral centers by 1H NMR spectroscopy, the following stereoisomers of dipeptide derivatives as guests were derived from the corresponding D- and L-amino acids (Fig. 3).
Their chemical structures are characterized by 1H NMR, 13C NMR, IR and HRMS methods. However, some protons cannot be clearly assigned on this NMR evidence alone, due to close chemical shift values, almost identical coupling constants and similar peak shapes. Therefore, 1H–1H COSY and 1H–13C HSQC spectra were measured and the assignments of the disputed protons were properly determined on the basis of their correlated signals.
As a typical example, the assignments of some related protons of G4-1 were clarified by 1H–1H COSY and 1H–13C HSQC. In the 1H NMR spectrum of G4-1, signals of the protons of PhCH2 and BnCH groups of phenylalanine residue of G4-1 are easily determined according to their chemical shift values, coupling constants and split peak shapes. However, the protons with chemical shift values at 4.93 and 5.40 ppm cannot be properly assigned owning to their same coupling constants and peak shapes (d, J = 6.96 Hz). Nevertheless, based on the correlation of their 1H–1H COSY and 1H–13C HSQC features, they can be very clearly assigned to be PhCH (5.40 ppm) of the phenylglycine residue and NH proton (4.93 ppm) of the TsNH group of N-Ts-phenylalanine residue. All relevant 1H–1H COSY and 1H–13C HSQC spectra are available in the ESI.†
To evaluate chiral discriminating properties of TAMCSAs 1a–1c, first, the 1H NMR spectrum of (±)-G1 (G1-1 (○) and G1-2 (●)) was measured in the presence of TAMCSA 1b. The result shows that the 1H NMR signals of the corresponding protons for the PhCH2, PhCH2O, TsNH, CONH and ArH groups of (±)-G1 were split and their nonequivalent chemical shifts (ΔΔδ) were found to be 0.013, 0.020, 0.012, 0.013, 0.151, 0.081 and 0.020 ppm (Fig. 4).
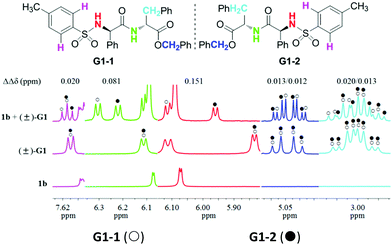 | ||
Fig. 4 Overlaid 1H NMR spectra (400 MHz) of (±)-G1 in the presence of TAMCSA 1b (TAMCSA 1b/(±)-G1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mM) in CDCl3, free (±)-G1 and free TAMCSA 1b from top to bottom. 1, 10 mM) in CDCl3, free (±)-G1 and free TAMCSA 1b from top to bottom. | ||
Among them, ΔΔδ of the NH proton for the TsNH group was demonstrated to provide the maximum value (up to 0.151 ppm). Two protons belonging to PhCH2 or PhCH2O groups, with a subtle difference in stereochemistry, are also differentiated. The aforementioned results suggest that strong hydrogen bonding interactions may exist in diastereomeric complexes of TAMCSA 1b with (±)-G1. Consequently, under the same conditions, 1H NMR spectra of G1-1 and G1-2 were also measured, respectively. Assignment of the two enantiomers of (±)-G1 was easily determined by comparing their chemical shift values, coupling constants and peak shapes. These preliminary results suggest that TAMCSA 1b has excellent binding properties towards chiral discrimination of (±)-G1.
To obtain better baseline resolution for chiral discrimination, the following chiral discriminating conditions were optimized. Firstly, 1H NMR spectra of (±)-G1 with TAMCSA 1b were performed in different deuterated solvents such as CDCl3, CDCl3 containing 10% C6D6, CD3COCD3, CD3OD and DMSO-d6, respectively. The changes of ΔΔδ of the selected protons without any interfering signals are summarized in Table 1.
| Solvent | PhCH2O | TsNH | CONH | ArH |
|---|---|---|---|---|
a Concentrations of (±)-G1 and TAMCSA 1b are 10 mM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1).
|
||||
| CDCl3 | 0.012/0.013 | 0.151 | 0.081 | 0.020 |
| CDCl3/C6D6 (10%) | 0.003/0.004 | 0.060 | 0.035 | 0.007 |
| CDCl3/CD3COCD3 (10%) | 0.000/0.000 | 0.038 | 0.018 | 0.000 |
| CDCl3/CD3OD (10%) | 0.000/0.000 | — | — | 0.000 |
| CDCl3/DMSO-d6 (10%) | 0.000/0.000 | — | — | 0.000 |
As shown in Table 1, upon enhancement of solvent polarity, the intermolecular interactions between enantiomers of (±)-G1 and TAMCSA 1b were weakened and even disappeared. The results suggest that CDCl3 is more suitable for chiral discrimination by 1H NMR spectroscopy.
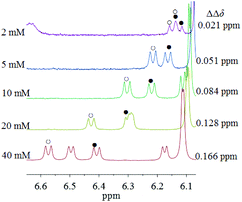
Secondly, samples of (±)-G1 with different concentrations were prepared in the presence of TAMCSA 1b in CDCl3, and their 1H NMR spectra were measured. Overlaid 1H NMR spectra featuring the NH proton of the CONH group are shown in Fig. 5.
As the concentration gradually increase, the ΔΔδ values of the NH proton of the CONH group exhibit a gradually increasing trend from 0.021 (2 mM) to 0.166 ppm (40 mM). Based on general requirements for concentration in 1H NMR spectroscopy, and common solubilities of hosts and guests, a concentration of 10 mM was used for chiral discrimination. It should be clarified that both a double peak at 6.17 ppm and another double peak between 6.40 and 6.58 ppm at 40 mM derive from 1H NMR signals of NH protons of the TsNH group of a pair of enantiomers of (±)-G1.
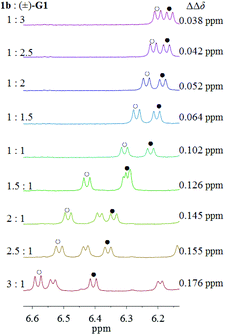
Lastly, upon increasing the molar ratio of TAMCSA 1b, the ΔΔδ values of the NH protons of the CONH group of (±)-G1 were showing a gradually increasing trend. The largest change of ΔΔδ was found to be 0.038 ppm upon varying the molar ratios of TAMCSA 1b over (±)-G1 from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 to 1
1.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 6). Based on these results, the 1
1 (Fig. 6). Based on these results, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of (±)-G1 with TAMCSAs 1b was used for chiral discrimination.
1 molar ratio of (±)-G1 with TAMCSAs 1b was used for chiral discrimination.
Upon completion of the aforementioned optimization study, and in order to obtain further evidence to demonstrate the chiral discriminating ability of TAMCSAs 1a–1c, 1H NMR spectra were measured for all (±)-G1–G8 samples prepared in the presence of TAMCSAs 1a–1c, with the exception of (±)-G1 that was prepared only in the presence of TAMCSAs 1b. The 1H NMR spectra show that signals of various types of key protons belonging to (±)-G1–G8 exhibit splitting. Furthermore, TAMCSAs 1a–1c provide multiple detection capabilities for chiral discrimination in most cases. Among them, ΔΔδ value of the NH proton (TsNH group) of (±)-G8 is found to exhibit a maximum (0.486 ppm) in the presence of TAMCSA 1a. In particular, 1H NMR signals due to eight types of protons of (±)-G5 can be differentiated in the presence of TAMCSAs 1a–1c. 1H NMR spectra of the corresponding protons of (±)-G5 in the presence of TAMCSA 1a are shown in different colors (Fig. 7).
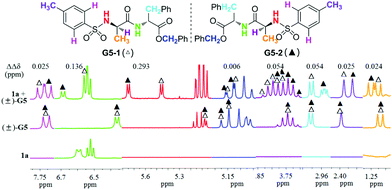 | ||
Fig. 7 Overlaid 1H NMR spectra (400 MHz) of (±)-G5 in the presence of TAMCSA 1a (TAMCSA 1a/(±)-G5 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mM), free (±)-G5 and free TAMCSA 1a from top to bottom. 1, 10 mM), free (±)-G5 and free TAMCSA 1a from top to bottom. | ||
As shown in Fig. 7, the ΔΔδ values of protons belonging to CH3CH, PhCH3, PhCH2CH, CH3CH, PhCH2O, TsNH, CONH and ArH groups of the (±)-G5 enantiomers are found to be 0.024, 0.025, 0.054, 0.054, 0.006, 0.293, 0.136 and 0.025 ppm in sequence. In particular, two aromatic hydrogens (ArH, pink) of the benzene ring (Ts group) were discriminated as well. This indicates that π–π stacking interactions may operate in the diastereoisomeric complexes of (±)-G5 with TAMCSA 1a.
To further test the intermolecular non-covalent interactions, the association constants of complexes of G5-1 and G5-2 with TAMCSA 1a were calculated by the nonlinear least-squares methods, respectively.15 The detailed results are summarized in Table 2.
Following measurements of the enantiomeric discrimination patterns noted above, 1H NMR spectra of each enantiomer of G1–G8 with TAMCSAs 1a–1c were obtained under the same conditions. The assignments of enantiomers of (±)-G1–G8 were determined by comparing their chemical shift values, coupling constants and peak shapes. As an example, the partial 1H NMR spectra and nonequivalent chemical shifts of the NH proton belonging to the TsNH group of (±)-G1–G8 are demonstrated in Table 3, with the exception of (±)-G3 in the presence of TAMCSA 1a and (±)-G6 in the presence of TAMCSAs 1b and 1c, since the 1H NMR signal of the corresponding NH proton (TsNH group) exhibit interference by signals from other protons or are not discriminated (Table 3). Nonequivalent chemical shifts of other discriminated protons of (±)-G1–G8 are summarized in Table 4. Their partial 1H NMR spectra are available in the ESI.†
| TAMCSA/guest/ΔΔδ | Spectrab,d | Spectrac,d | TAMCSA/guest/ΔΔδ | Spectrab,d | Spectrac,d |
|---|---|---|---|---|---|
a ΔΔδ = |Δδ1 − Δδ2|, Δδ1 = |δ1 − δfree|, Δδ2 = |δ2 − δfree|, the numbers “1 and 2” present two enantiomers of (±)-G1-8, respectively.
b Partial 1H NMR spectra of NH protons of Ts group of (±)-G1-8 (10 × 10−3 M) in the presence of TAMCSAs 1a–1c, respectively, H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G = 1 G = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
c Overlaid partial 1H NMR spectra of NH protons of Ts group of a single enantiomer of (±)-G1-8 (10 × 10−3 M) under the same conditions noted above, H 1.
c Overlaid partial 1H NMR spectra of NH protons of Ts group of a single enantiomer of (±)-G1-8 (10 × 10−3 M) under the same conditions noted above, H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G = 1 G = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
d The following signs stand for the corresponding stereoisomers: G1-1 (○), G1-2 (●); G2-1 (□), G2-2 (■); G3-1 (▽), G3-2 (▼); G4-1 (◊), G4-2 (◆); G5-1 (△), G5-2 (▲); G6-1 (☆), G6-2 (★); G7-1 (♤), G7-2 (♠); G8-1 (♡), G8-2 (♥). 1.
d The following signs stand for the corresponding stereoisomers: G1-1 (○), G1-2 (●); G2-1 (□), G2-2 (■); G3-1 (▽), G3-2 (▼); G4-1 (◊), G4-2 (◆); G5-1 (△), G5-2 (▲); G6-1 (☆), G6-2 (★); G7-1 (♤), G7-2 (♠); G8-1 (♡), G8-2 (♥).
|
|||||
| 1a/(±)-G1/0.154 |
|
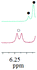
|
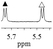
1a/(±)-G5/0.293 |
|
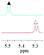
|
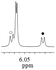
| 1b/(±)-G1/0.151 |
|
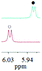
|
1b/(±)-G5/0.133 |
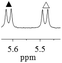
|

|
| 1c/(±)-G1/0.160 |

|
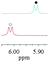
|
1c/(±)-G5/0.132 |
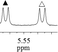
|

|
| 1a/(±)-G2/0.142 |
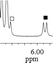
|

|
1a/(±)-G6/0.048 |
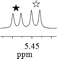
|
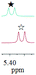
|
| 1b/(±)-G2/0.143 |

|

|
1a/(±)-G7/0.141 |

|
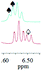
|
| 1c/(±)-G2/0.140 |

|

|
1b/(±)-G7/0.104 |
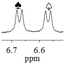
|

|
| 1b/(±)-G3/0.060 |

|
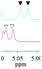
|
1c/(±)-G7/0.105 |

|

|
| 1c/(±)-G3/0.059 |

|

|
1a/(±)-G8/0.486 |
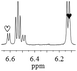
|

|
| 1a/(±)-G4/0.199 |

|

|
1b/(±)-G8/0.425 |
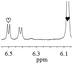
|

|
| 1b/(±)-G4/0.243 |

|

|
1c/(±)-G8/0.432 |
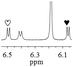
|
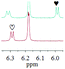
|
| 1c/(±)-G4/0.242 |

|

|
|||
| TAMCSA/guest | Proton | ΔΔδ (ppm) | TAMCSA/guest | Proton | ΔΔδ (ppm) |
|---|---|---|---|---|---|
a Nonequivalent chemical shifts (ΔΔδ, ppm) of (±)-G1–G8 (10 × 10−3 M) in the presence of TAMCSAs 1a–1c, respectively, H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G = 1 G = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
|||||
| 1a/(±)-G1 | PhCH2 | 0.021 | ArH | 0.025 | |
| 0.032 | 1b/(±)-G5 | CH 3 | 0.014 | ||
| PhCH2O | 0.011 | PhCH3 | 0.015 | ||
| 0.012 | PhCH2 | 0.029 | |||
| CONH | 0.066 | CH3CH | 0.048 | ||
| ArH | 0.021 | PhCH2O | 0.007 | ||
| 1b/(±)-G1 | PhCH2 | 0.013 | 0.009 | ||
| 0.020 | CONH | 0.075 | |||
| PhCH2O | 0.012 | ArH | 0.019 | ||
| 0.013 | 1c/(±)-G5 | CH 3 | 0.014 | ||
| CONH | 0.081 | PhCH3 | 0.014 | ||
| ArH | 0.020 | PhCH2 | 0.029 | ||
| 1c/(±)-G1 | PhCH2 | 0.014 | CH3CH | 0.049 | |
| 0.022 | PhCH2O | 0.008 | |||
| PhCH2O | 0.013 | 0.010 | |||
| 0.013 | CONH | 0.072 | |||
| CONH | 0.089 | ArH | 0.017 | ||
| ArH | 0.021 | 1a/(±)-G6 | (CH3)2CH | 0.011 | |
| 1a/(±)-G2 | PhCH2 | 0.013 | 0.009 | ||
| 0.013 | PhCH2 | 0.025 | |||
| PhCH2O | 0.004 | 0.014 | |||
| 0.004 | (CH3)2CHCH | 0.057 | |||
| CONH | 0.034 | PhCH2O | 0.010 | ||
| ArH | 0.008 | CONH | 0.089 | ||
| 1b/(±)-G2 | PhCH2 | 0.008 | ArH | 0.021 | |
| 0.007 | 1b/(±)-G6 | (CH3)2CH | 0.007 | ||
| CONH | 0.035 | 0.005 | |||
| ArH | 0.007 | PhCH2 | 0.015 | ||
| 1c/(±)-G2 | PhCH2 | 0.007 | (CH3)2CHCH | 0.048 | |
| 0.008 | PhCH2O | 0.010 | |||
| CONH | 0.073 | CONH | 0.081 | ||
| ArH | 0.006 | ArH | 0.017 | ||
| 1a/(±)-G3 | CH 3 | 0.009 | 1c/(±)-G6 | (CH3)2CH | 0.007 |
| PhCH2 | 0.009 | 0.005 | |||
| 0.014 | PhCH2 | 0.014 | |||
| PhCH2O | 0.004 | (CH3)2CHCH | 0.043 | ||
| PhCH | 0.008 | PhCH2O | 0.008 | ||
| ArH | 0.006 | CONH | 0.074 | ||
| 1b/(±)-G3 | CH 3 | 0.011 | ArH | 0.015 | |
| PhCH2 | 0.015 | 1a/(±)-G7 | PhCH3 | 0.022 | |
| 0.017 | PhCH2 | 0.017 | |||
| PhCH2O | 0.007 | 0.035 | |||
| PhCH | 0.003 | PhCH2O | 0.008 | ||
| ArH | 0.006 | 0.008 | |||
| 1c/(±)-G3 | CH 3 | 0.011 | ArH | 0.005 | |
| PhCH2 | 0.015 | 1b/(±)-G7 | PhCH3 | 0.012 | |
| 0.016 | PhCH2O | 0.007 | |||
| PhCH2O | 0.006 | 1c/(±)-G7 | PhCH3 | 0.012 | |
| PhCH | 0.004 | PhCH2O | 0.007 | ||
| ArH | 0.007 | 1a/(±)-G8 | CH 3 | 0.100 | |
| 1a/(±)-G4 | PhCH2O | 0.052 | PhCH | 0.060 | |
| 0.071 | PhCH2O | 0.011 | |||
| PhCH | 0.047 | CONH | 0.250 | ||
| 1b/(±)-G4 | PhCH2O | 0.005 | ArH | 0.055 | |
| 0.029 | 1b/(±)-G8 | CH 3 | 0.077 | ||
| PhCH | 0.031 | PhCH | 0.058 | ||
| 1c/(±)-G4 | PhCH2O | 0.006 | PhCH2O | 0.009 | |
| 0.029 | CONH | 0.247 | |||
| PhCH | 0.031 | ArH | 0.052 | ||
| 1a/(±)-G5 | CH 3 | 0.024 | 1c/(±)-G8 | CH 3 | 0.078 |
| PhCH3 | 0.025 | PhCH | 0.056 | ||
| PhCH2 | 0.054 | PhCH2O | 0.009 | ||
| CH3CH | 0.054 | CONH | 0.253 | ||
| PhCH2O | 0.006 | ArH | 0.052 | ||
| CONH | 0.136 | ||||
As shown in Table 4, an unprecedented number of different types of protons from the same chiral analyte can be discriminated by TAMCSAs 1a–1c using 1H NMR spectroscopy. These results show that TAMCSAs 1a–1c are highly effective chiral solvating agents with respect to the dipeptide derivatives (±)-G1–G8.
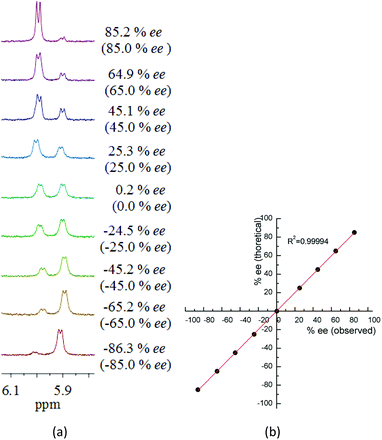
Now that TAMCSAs 1a–1c have been established to demonstrate excellent chiral discriminating abilities, their practical applicability was tested in determining enantiomeric excess (ee) of dipeptide derivatives with different optical compositions. Samples containing G1-1 with 85%, 65%, 45%, 25%, 0%, −25%, −45%, −65% and −85% ee were prepared in the presence of TAMCSA 1c and then their 1H NMR spectra were recorded. The enantiomeric excesses (ee) of all samples were accurately obtained based on the integration of 1H NMR signals of the NH protons (TsNH group) of enantiomers G1-1 and G1-2 (Fig. 8).
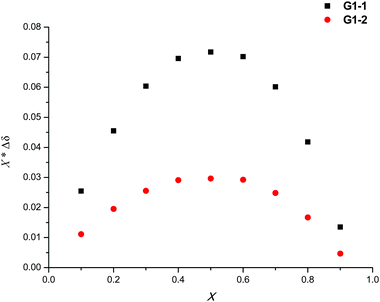
Additionally, in order to further investigate chiral discriminating behavior, Job plots of (±)-G1 were performed in the presence of TAMCSA 1c. The Job plots of (±)-G1 exhibited a maximum value (X*Δδ = 0.042 ppm) at a molar fraction of X = 0.5, which suggests that a diastereoisomer complex with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was established between (±)-G1 and TAMCSA 1c (Fig. 9).
1 stoichiometry was established between (±)-G1 and TAMCSA 1c (Fig. 9).
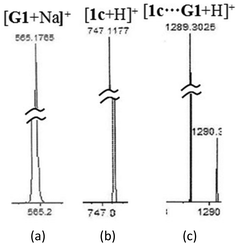
To verify the results noted above, the stoichiometric interaction of (±)-G1 with TAMCSA 1c was evaluated by electrospray ionization (ESI) mass spectroscopy. The partial ESI mass spectrum of TAMCSA 1c and (±)-G1 exhibit formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex [1c + G1 + H]+ (m/z 1289.3025). Partial and expanded ESI mass spectra derived from the same spectrum are shown in Fig. 10. The full ESI mass spectrum of (±)-G1 and TAMCSA 1c is available in the ESI.†
1 complex [1c + G1 + H]+ (m/z 1289.3025). Partial and expanded ESI mass spectra derived from the same spectrum are shown in Fig. 10. The full ESI mass spectrum of (±)-G1 and TAMCSA 1c is available in the ESI.†
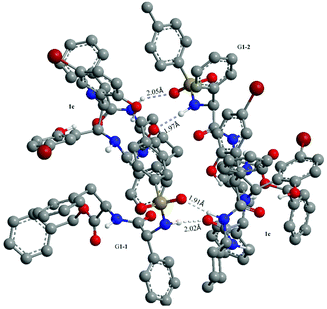
Finally, to better understand the chiral discriminating behavior between (±)-G1 and TAMCSA 1c, theoretical calculations were carried out using an AM1 model by Gaussian 03 program.16 The proposed model suggests that a pair of diastereoisomer complexes of (±)-G1 with TAMCSA 1c are formed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. The proposed model exhibits the distances of hydrogen bonds (S
1 stoichiometry. The proposed model exhibits the distances of hydrogen bonds (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HNCO and NHCO⋯HNSO2) of G1-1 and G1-2 with TAMCSA 1c as 1.91 and 2.02, 2.05 and 1.97 Å, respectively. The models separated from the following proposed binding model are available in the ESI.† The proposed binding model is shown in Fig. 11.
O⋯HNCO and NHCO⋯HNSO2) of G1-1 and G1-2 with TAMCSA 1c as 1.91 and 2.02, 2.05 and 1.97 Å, respectively. The models separated from the following proposed binding model are available in the ESI.† The proposed binding model is shown in Fig. 11.
Conclusions
In summary, we have developed a new class of tetraaza macrocyclic chiral solvating agents (TAMCSAs) 1a–1c with two amide, two amino and two phenolic hydroxyl groups, featuring C2-symmetry and a 12-membered cavity as a framework for chiral discrimination. All enantiomers of the dipeptide derivatives with two chiral centers (±)-G1–G8 were successfully discriminated by the corresponding 1H NMR signals of various types of protons in the presence of TAMCSAs 1a–1c. Enantiomeric excesses (ee) of G1 with different optical compositions were calculated based on the integration of 1H NMR signals of NH protons of the TsNH group of enantiomers G1-1 and G1-2 in the presence of TAMCSA 1c. The results have proved that the unique structural features of TAMCSAs 1a–1c are enormously helpful for promoting chiral discrimination. The chiral discriminating behavior has been evaluated by means of Job plots, ESI mass spectra and a theoretically proposed model of (±)-G1 with TAMCSA 1c. In addition, the association constants of enantiomers of (±)-G5 with TAMCSA 1a were calculated by the nonlinear curve-fitting method. In conclusion, TAMCSAs 1a–1c have exhibited strong binding properties, better baseline resolution and multiple detection windows for chiral discrimination. These results demonstrate that TAMCSAs 1a–1c are especially suitable for chiral discrimination of these dipeptide derivatives by 1H NMR spectroscopy.Acknowledgements
This work was supported by The Beijing Municipal Commission of Education. Work in the Missouri lab (P. S.) is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R15GM117508.Notes and references
-
(a) D. Parker, Chem. Rev., 1991, 91, 1441–1457 CrossRef CAS
; (b) T. Kurtán, N. Nesnas, F. E. Koehn, Y. Q. Li, K. Nakanishi and N. Berova, J. Am. Chem. Soc., 2001, 123, 5974–5982 CrossRef
; (c) J. M. Seco, E. Quiñoá and R. Riguera, Chem. Rev., 2004, 104, 17–117 CrossRef CAS
; (d) A. Lakshmipriya, S. R. Chaudhari and N. Suryaprakash, Chem. Commun., 2015, 51, 13492–13495 RSC
; (e) Y. C. Zhao and T. M. Swager, J. Am. Chem. Soc., 2015, 137, 3221–3224 CrossRef CAS PubMed
; (f) M. S. Seo and H. Kim, J. Am. Chem. Soc., 2015, 137, 14190–14195 CrossRef CAS PubMed
.
-
(a) M. Kaik, J. Gajewy, J. Grajewski and J. Gawronski, Chirality, 2008, 20, 301–306 CrossRef CAS PubMed
; (b) T. J. Wenzel and C. D. Chisholm, Chirality, 2011, 23, 190–214 CrossRef CAS PubMed
; (c) N. V. Orlov and V. P. Ananikov, Chem. Commun., 2010, 46, 3212–3214 RSC
; (d) S. R. Chaudhari and N. Suryaprakash, J. Org. Chem., 2012, 77, 648–651 CrossRef CAS PubMed
.
-
(a) T. J. Wenzel, B. E. Freeman, D. C. Sek, J. J. Zopf, T. Nakamura, J. Yongzhu, K. Hirose and Y. Tobe, Anal. Bioanal. Chem., 2004, 378, 1536–1547 CrossRef CAS PubMed
; (b) K. Omata, M. Fujioka, K. Kabuto and Y. Sasaki, Chem. Commun., 2008, 4903–4905 RSC
.
-
(a) D. Yang, X. Li, Y. F. Fan and D. W. Zhang, J. Am. Chem. Soc., 2005, 127, 7996–7997 CrossRef CAS PubMed
; (b) F. N. Ma, L. Ai, X. M. Shen and C. Zhang, Org. Lett., 2007, 9, 125–127 CrossRef CAS PubMed
; (c) Q. Z. Ma, M. S. Ma, H. Y. Tian, X. X. Ye, H. P. Xiao, L. H. Chen and X. X. Lei, Org. Lett., 2012, 14, 5813–5815 CrossRef CAS PubMed
.
-
(a) T. Ema, D. Tanida and T. Sakai, J. Am. Chem. Soc., 2007, 129, 10591–10596 CrossRef CAS PubMed
; (b) T. P. Quinn, P. D. Atwood, J. M. Tanski and T. F. Moore, J. Org. Chem., 2011, 76, 10020–10030 CrossRef CAS PubMed
; (c) H. N. Naziroglu, M. Durmaz, S. Bozkurt and A. Sirit, Chirality, 2011, 23, 463–471 CrossRef CAS PubMed
; (d) A. Nemes, T. Csóka, S. Béni, V. Farkas, J. Rábai and D. Szabó, J. Org. Chem., 2015, 80, 6267–6274 CrossRef CAS PubMed
.
-
(a)
G. R. Weisman, in Asymmetric Synthesis, ed. J. D. Morrison, Academic Press, New York, 1983, vol. 1 Search PubMed
; (b) R. R. Fraser, in Asymmetric Synthesis, ed. J. D. Morrison, Academic Press, New York, 1983, vol. 1 Search PubMed
; (c) T. J. Wenzel and J. D. Wilcox, Chirality, 2003, 15, 256–270 CrossRef CAS PubMed
; (d) H. Ishii, Y. Chen, R. A. Miller, S. Karady, K. Nakanishi and N. Berova, Chirality, 2005, 17, 305–315 CrossRef CAS PubMed
; (e) M. Kurosu and K. Li, Org. Lett., 2009, 11, 911–914 CrossRef CAS PubMed
; (f) G. L. Bian, S. W. Yang, H. Y. Huang, H. Zong, L. Song, H. J. Fan and X. Q. Sun, Chem. Soc., 2016, 7, 932–938 CAS
.
-
(a) F. Cuevas, P. Ballester and M. A. Pericàs, Org. Lett., 2005, 7, 5485–5487 CrossRef CAS PubMed
; (b) S. Kim and K. Choi, Eur. J. Org. Chem., 2011, 4747–4750 CAS
; (c) C. Wolf, A. M. Cook and J. E. Dannatt, Tetrahedron: Asymmetry, 2014, 25, 163–169 CrossRef CAS
; (d) J. Kannappan, N. Jain and A. V. Bedekar, Tetrahedron: Asymmetry, 2015, 26, 1102–1107 CrossRef CAS
.
-
(a) M. V. Rekharsky, H. Yamamura, M. Kawai and Y. Inoue, J. Org. Chem., 2003, 68, 5228–5235 CrossRef CAS PubMed
; (b) T. Ishizu, H. Tsutsumi and A. Yokoyama, Tetrahedron Lett., 2015, 56, 1111–1114 CrossRef CAS
.
-
(a) Y. Mei, P. Dissanayake and M. J. Allen, J. Am. Chem. Soc., 2010, 132, 12871–12873 CrossRef CAS PubMed
; (b) H. L. Cui, J. R. Huang, J. Lei, Z. F. Wang, S. Chen, L. Wu and Y. C. Chen, Org. Lett., 2010, 12, 720–723 CrossRef CAS PubMed
; (c) P. Daka, Z. H. Xu, A. Alexa and H. Wang, Chem. Commun., 2011, 47, 224–226 RSC
; (d) K. Lang, J. Park and S. Hong, Angew. Chem., Int. Ed., 2012, 51, 1620–1624 CrossRef CAS PubMed
; (e) Y. Shimoda, T. Kubo, M. Sugiura, S. Kotani and M. Nakajima, Angew. Chem., Int. Ed., 2013, 52, 3461–3464 CrossRef CAS PubMed
; (f) G. L. Bian, H. J. Fan, H. Y. Huang, S. W. Yang, H. Zong and L. Song, Org. Lett., 2015, 17, 1369–1372 CrossRef CAS PubMed
; (g) M. Gangar, H. M. Sandeep Goyal, M. N. Mungalpara and V. A. Nair, Tetrahedron Lett., 2016, 57, 3462–3467 CrossRef CAS
.
- C. Bombelli, S. Borocci, F. Lupi, G. Mancini, L. Mannina, A. L. Segre and S. Viel, J. Am. Chem. Soc., 2004, 126, 13354–13362 CrossRef CAS PubMed
.
-
(a) N. M. Maier, P. Franco and W. Lindner, J. Chromatogr., A, 2001, 906, 3–33 CrossRef CAS
; (b) H. N. Naziroglu, M. Durmaz, S. Bozkurt and A. Sirit, Chirality, 2011, 23, 463–471 CrossRef CAS PubMed
.
-
(a) V. F. Cruzat, L. C. Pantaleão Jr., J. Donato Jr., P. I. Homem de Bittencourt and J. Tirapegui, J. Nutr. Biochem., 2014, 25, 345–352 CrossRef CAS PubMed
; (b) W. P. McCormack, J. R. Hoffman, G. J. Pruna, A. R. Jajtner, J. R. Townsend, J. R. Stout, M. S. Fragala and D. H. Fukuda, J. Am. Coll. Nutr., 2015, 34, 488–496 CrossRef CAS PubMed
.
- J. C. Melchior, D. Rigaud, N. Colas-Linhart, A. Petiet, A. Girard and M. Apfelbaum, Physiol. Behav., 1991, 50, 941–944 CrossRef CAS PubMed
.
- G. P. Gao, C. X. Lv, Q. J. Li, L. Ai and J. X. Zhang, Tetrahedron Lett., 2015, 56, 6742–6746 CrossRef CAS
.
-
(a) Y. Inoue, K. Yamamoto, T. Wada, S. Everitt, X. M. Gao, Z. J. Hou, S. K. Jiang and H. M. Wu, J. Chem. Soc., Perkin Trans. 2, 1998, 1807–1816 RSC
; (b) J. Z. Fan, Y. Chen, D. R. Cao, Y. W. Yang, X. S. Jia and C. J. Li, RSC Adv., 2014, 4, 4330–4333 RSC
.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Gaussian, Inc., Wallingford CT, 2004 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of TAMCSAs 1a–1c and enantiomers of dipeptide derivatives with two chiral centers G1–8, experimental detail and original NMR spectra of chiral discrimination. CCDC 1007044, 1007045 and 1007047. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00521g |
| This journal is © the Partner Organisations 2016 |