Rh(i)-Catalyzed coupling of 2-bromoethyl aryldiazoacetates with tertiary propargyl alcohols through carbene migratory insertion†
Abstract
A Rh(I)-catalyzed cross-coupling of 2-bromoethyl aryldiazoacetates with tertiary propargyl alcohols has been successfully achieved. This reaction follows a reaction sequence which involves C–C bond cleavage, Rh(I) carbene formation, carbene migratory insertion and nucleophilic substitution to achieve the construction of C(sp2)–C(sp) and C–O bonds. The products feature both enyne and ketene acetal moieties, which have been widely used as building blocks in organic synthesis and polymer science.
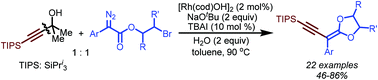
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2016

 Please wait while we load your content...
Please wait while we load your content...