Manganese-catalyzed ring-opening chlorination of cyclobutanols: regiospecific synthesis of γ-chloroketones†
Leitao
Huan
a and
Chen
Zhu
*ab
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu 215123, China. E-mail: chzhu@suda.edu.cn
bKey Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 5th September 2016
Abstract
We disclose an efficient manganese-catalyzed ring-opening chlorination of cyclobutanols. This reaction has a broad substrate scope, affording a variety of γ-chloro alkyl ketones and medium-sized benzocyclic chlorides in synthetically useful yields and unique regioselectivities. The merits including mild reaction conditions and employment of inexpensive catalysts and reagents make it a practical approach for the production of γ-chlorinated ketones.
Introduction
Chlorinated alkyl ketones have long been used as versatile key building blocks in organic and medicinal synthesis.1 Therefore, the convenient acquisition of chlorinated alkyl ketones is of high importance in multidisciplinary fields. Generally, the synthesis of α-chloroketones is achieved by the electrophilic chlorination of a ketone enolate;2 the β-chloroketones can be obtained through the Michael addition of chloride to α,β-unsaturated ketones,3 the Baylis–Hillman reaction,4 and the direct chlorofunctionalization of carbonyl-activated alkenes.5 However, the construction of γ-chloroketones is relatively less investigated. A conventional approach to γ-chloroketones relies on the Friedel–Craft reaction of electron-rich arenes with moisture-sensitive 4-chlorobutyryl chloride in the presence of stoichiometric amounts of strong Lewis acid.6 Apparently, the electron-deficient and meta-substituted products cannot be prepared by this method, and many susceptible functional groups are also incompatible with the harsh reaction conditions. Thus, the development of a mild and efficient method to produce a broad diversity of γ-chloroketones is still desirable.Cyclobutanols have been proven to be privileged precursors for the synthesis of γ-functionalized ketones by breaking the cyclic C–C bond.7,8 Inspired by the seminar work about the single-electron oxidation of cyclobutanol to enable ring opening by the use of stoichiometric amounts of the high-valent metal oxidants (e.g., CAN and LTA),9 we have developed a sequence of catalytic ring-opening functionalization of cyclobutanols to yield γ-fluoro,10 azido,11 cyano and alkynyl,12 thio,13 and hydrazine-substituted alkyl ketones by means of silver or manganese catalysis.14
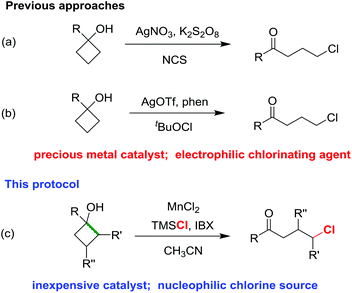
Recently, we firstly disclosed the ring-opening chlorination of cyclobutanols in the presence of a silver catalyst and NCS as the chlorine source (Scheme 1a).15 Later, Zhang and co-workers revealed the silver-catalyzed chlorination of cyclobutanols by using tBuOCl (Scheme 1b).16 In both cases, the precious metal catalysts and electrophilic chlorinating reagents were harnessed. Herein, we report an utterly different approach of ring-opening chlorination of cyclobutanols in the presence of an inexpensive MnCl2 catalyst and nucleophilic TMSCl (Scheme 1c). The reaction has a broad substrate scope, affording a variety of synthetically useful γ-chloro alkyl ketones and medium-sized benzocyclic chlorides with exclusive regioselectivities under mild reaction conditions.
Results and discussion
Experimental study
The investigations of ring-opening chlorination of cyclobutanols began with the extensive reaction parameter survey (Table 1). With MnCl2 as the catalyst and TMSCl as the chlorine source, a set of oxidants were examined. While many oxidants such as H2O2, K2S2O8, mCPBA, and DTBP were inefficient (entries 1–4), TBHP delivered a good yield (75%, entry 5). Further examination of oxidants demonstrated that hypervalent iodine reagents were beneficial for this conversion in general (entries 6–9), and among them, IBX improved the isolated yield to 87% (entry 9). Later, many common organic solvents were also examined, implying that acetonitrile was the most suitable solvent for the reaction (entries 10–14). Variation of the MnCl2 catalyst to Mn(OAc)3 or Mn(acac)3 slightly decreased the reaction outcomes (entries 15 and 16). Reducing the amounts of TMSCl (3 equiv.) also compromised the reaction yields (entries 17 and 18). Performing the reaction in air gave lower yield than that under N2 (entry 19).| Entry | Oxidant | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.20 mmol), TMSCl (0.60 mmol, 3.0 equiv.), MnCl2·4H2O (0.02 mmol, 0.1 equiv.), and oxidant (0.40 mmol, 2.0 equiv.) in solvent (1.0 mL, 0.2 M) at 50 °C, N2. b Yields of isolated products. c 25 °C. d Mn(OAc)3·2H2O. e Mn(acac)3. f 2 equiv. TMSCl. g 1.5 equiv. TMSCl. h In air. | |||
| 1 | H2O2 | CH3CN | 13 |
| 2 | K2S2O8 | CH3CN | <10 |
| 3 | mCPBA | CH3CN | <10 |
| 4 | DTBP | CH3CN | 21 |
| 5 | TBHP | CH3CN | 75 |
| 6c | PIDA | CH3CN | 67 |
| 7 | PhIO | CH3CN | 70 |
| 8 | BI-OH | CH3CN | 76 |
| 9 | IBX | CH3CN | 87 |
| 10 | IBX | DCE | 75 |
| 11 | IBX | THF | 62 |
| 12 | IBX | Dioxane | 65 |
| 13 | IBX | Toluene | 52 |
| 14 | IBX | DMF | 72 |
| 15d | IBX | CH3CN | 83 |
| 16e | IBX | CH3CN | 81 |
| 17f | IBX | CH3CN | 81 |
| 18g | IBX | CH3CN | 77 |
| 19h | IBX | CH3CN | 67 |
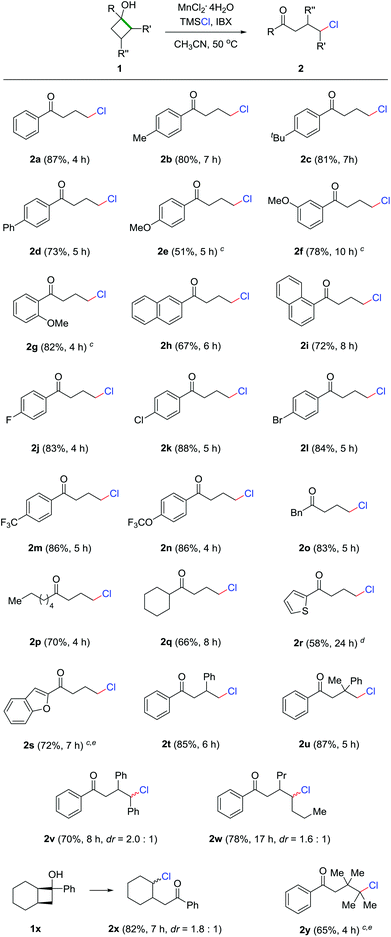
With the optimized reaction conditions in hand, we set out to evaluate the generality of this protocol. The reaction demonstrated broad functionality tolerance where both electron-rich and deficient cyclobutanols were compatible with the reaction conditions, affording a diversity of γ-chlorinated ketones in synthetically useful yields (Scheme 2). Generally, phenyl cyclobutanols bearing electron-donating groups led to good reaction outcomes (2a–2d). However, para-methoxy substituted cyclobutanol unexpectedly generated product 2e in moderate yield, which might be attributed to the incompatibility of the 4-methoxybenzene moiety under the highly oxidative conditions. In contrast, meta- and ortho-methoxy substrates generated the corresponding products in good yields (2f and 2g). Naphthyl cyclobutanol was another apt substrate for the reaction (2h and 2i). Electron-deficient cyclobutanols also efficiently provided the γ-chlorinated ketones in good yields (2j–2n). Remarkably, the presence of aryl bromide in 2l could offer a platform for further modification by cross coupling. In addition to aryl cyclobutanols, alkyl and heteroaryl cyclobutanols were also readily converted into the corresponding products in useful yields (2o–2s). According to the Thorpe–Ingold effect,17 the cyclic skeleton could be significantly stabilized by multiple substitutions on the four-membered ring. Though challenging, the products 2t–2w were still generated in good yields. In the cases of 2v and 2w, most importantly, the chlorine was regioselectively introduced at the methine position, producing secondary alkyl chlorides. The example of 2x is noteworthy, as the chlorination only took place on the ring to give cyclic chloride as the sole product when a bicyclic substrate was employed. With the highly substituted and sterically congested cyclobutanol, tertiary chloride 2y was obtained in acceptable yield.
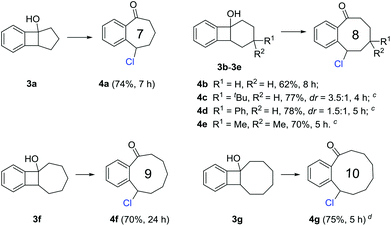
Medium-sized all-carbon rings are widely found in natural products and biologically active compounds, but their synthesis still remains difficult. Under the previous reaction conditions, a portfolio of benzocyclic chlorides ranging from seven- to ten-membered rings were readily obtained in synthetically useful yields (Scheme 3). These transformations could proceed at room temperature in general (3a, 3b, 3f, and 3g), but sometimes a higher temperature (50 °C) was required for the substrates bearing additional substituents on the cyclic framework (3c–3e). It might be anticipated that these products could be conveniently converted into other complex molecules by manipulation of the benzylic chloride.
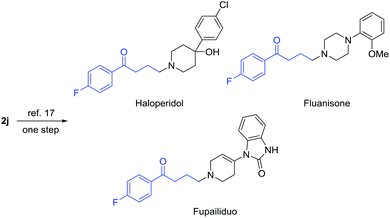
Of note, γ-chloroketones are privileged synthetic intermediates for the production of many drugs and biologically active compounds. For example, compound 2j can be readily converted into antipsychotic medications, e.g. haloperidol, fluanisone, and fupailiduo, by one-step nucleophilic substitution (Fig. 1).18
Proposed mechanism
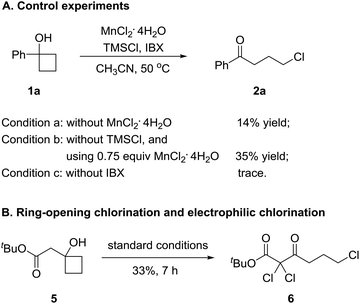
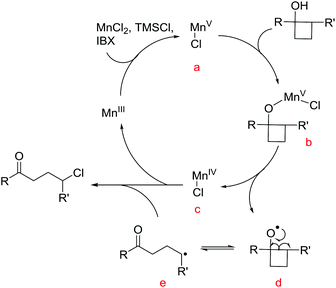
To gain insights into the manganese-catalyzed ring-opening chlorination and elucidate the possible mechanism, some experiments were carried out (Scheme 4). Initially, the reaction of 1a was performed in the absence of MnCl2 (Scheme 4A, condition a). The obtained low yield clearly implied the critical role of the manganese catalyst in the transformation. The reaction also proceeded by replacing TMSCl with a substoichiometric amount of MnCl2, which suggested that both TMSCl and MnCl2 served as the chlorine source (Scheme 4A, condition b). The reaction did not take place without IBX, showing that the oxidant was a requisite for the conversion (Scheme 4A, condition c). Then, we conducted the reaction with cyclobutanol 5 under the standard reaction conditions (Scheme 4B). The formation of 6 explicitly illustrated the in situ generation of electrophilic chlorinating species during the process.Based on the experimental observations and our previous realizations, a radical-mediated reaction mechanism was postulated (Fig. 2). Initially, the interaction between MnCl2, TMSCl, and the hypervalent iodine reagent led to the high-valent MnV–Cl species a,19 which could single-electron oxidize cyclobutanol to cyclobutoxy radical dvia complex b and concurrently released the MnIV–Cl species c. Tautomerization of radical d generated the open-chain alkyl radical e, which was subsequently intercepted by the MnIV–Cl species c to eventually furnish the γ-chlorinated ketones.
Conclusions
An efficient manganese-catalyzed ring-opening chlorination of cyclobutanols is described. The reaction demonstrated broad functionality tolerance, furnishing a range of γ-chloro alkyl ketones and medium-sized benzocyclic chlorides in synthetically useful yields and exclusive regioselectivities. The advantages of this protocol include mild reaction conditions and employment of inexpensive catalysts and reagents, making it a practical approach for the production of γ-chlorinated ketones.Experimental section
Cyclobutanol 1 or 3 (0.20 mmol, 1.0 equiv.), MnCl2·4H2O (0.02 mmol, 0.1 equiv.), and IBX (0.40 mmol, 2.0 equiv.) were loaded in a flask which was subjected to evacuation/flushing with nitrogen three times. CH3CN (1.0 mL) followed by TMSCl (0.60 mmol, 3.0 equiv.) was added to the mixture via a syringe and the mixture was then stirred at 50 °C (or 25 °C) until the starting material had been consumed as determined by TLC. The mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic extracts were washed by using brine, dried over Na2SO4, filtered, concentrated, and purified by flash column chromatography on silica gel (ethyl acetate/petroleum ether) to give the product 2 or 4.Conflict of interest
The authors declare no competing financial interests.Acknowledgements
C. Z. is grateful for the financial support from Soochow University, the National Natural Science Foundation of China (Grant no. 21402134), the Natural Science Foundation of Jiangsu Province (Grant no. BK20140306), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- For selected examples, see: (a) M. A. Iorio, T. Paszkowska Reymer and V. Frigeni, J. Med. Chem., 1987, 30, 1906 CrossRef CAS PubMed; (b) I. van Winjngaarden, C. G. Kruse, J. A. M. van der Heyden and M. Th. M. Tulip, J. Med. Chem., 1988, 31, 1934 CrossRef; (c) C.-A. Chen, Y. Jiang, K. Lu, I. Daniewska, C. G. Mazza, L. Negron, C. Forray, T. Parola, B. Li, L. G. Hegde, T. D. Wolinksy, D. A. Craig, R. Kong, J. M. Wetzel, K. Andersen and M. R. Marzabadi, J. Med. Chem., 2007, 50, 3883 CrossRef CAS PubMed.
- For selected examples, see: (a) D. Yang, Y.-L. Yan and B. Liu, J. Org. Chem., 2002, 67, 7429 CrossRef CAS PubMed; (b) R. Frantz, L. Hintermann, M. Perseghini, D. Broggini and A. Togni, Org. Lett., 2003, 5, 1709 CrossRef CAS PubMed; (c) C. Wang and J. Tunge, Chem. Commun., 2004, 23, 2694 RSC; (d) M. Marigo, S. Bachmann, N. Halland, A. Braunton and K. A. Jørgensen, Angew. Chem., Int. Ed., 2004, 43, 5507 CrossRef CAS PubMed; (e) M. Marigo, N. Kumaragurubaran and K. A. Jørgensen, Chem. – Eur. J., 2004, 10, 2133 CrossRef CAS PubMed; (f) F. Grein, A. C. Chen, D. Edwards and C. M. Crudden, J. Org. Chem., 2006, 71, 861 CrossRef CAS PubMed; (g) K. Shibatomi, Y. Soga, A. Narayama, I. Fujisawa and S. Iwasa, J. Am. Chem. Soc., 2012, 134, 9836 CrossRef CAS PubMed.
- For selected examples, see: (a) L. I. Smith and J. A. Sprung, J. Am. Chem. Soc., 1943, 65, 1276 CrossRef CAS; (b) V. L. Heasley, D. F. Shellhamer, T. L. Carter, D. E. Gipe, R. K. Gipe, R. C. Green, J. Hordeen, T. D. Rempel, D. H. Spaite and G. E. Heasley, Tetrahedron Lett., 1981, 22, 2467 CrossRef CAS; (c) J. N. Marx, Tetrahedron, 1983, 39, 1529 CrossRef CAS.
- For selected examples, see: (a) G. Li, J. Gao, H.-X. Wei and M. Enright, Org. Lett., 2000, 2, 617 CrossRef CAS PubMed; (b) M. Shi, J.-K. Jiang and Y.-S. Feng, Org. Lett., 2000, 2, 2397 CrossRef CAS PubMed; (c) M. Shi and Y.-S. Feng, J. Org. Chem., 2001, 66, 406 CrossRef CAS PubMed; (d) Z. Han, S. Uehira, H. Shinokubo and K. Oshima, J. Org. Chem., 2001, 66, 7854 CrossRef CAS PubMed.
- For selected examples, see: (a) S. Piettre, Z. Janousek, R. Merenyi and H. G. Viehe, Tetrahedron, 1985, 41, 2527 CrossRef CAS; (b) L. Engman, J. Org. Chem., 1988, 53, 4031 CrossRef CAS; (c) F. D'Onofrio, L. Parlanti and G. Piancatelli, Tetrahedron Lett., 1995, 36, 1929 CrossRef; (d) Y. Li, M. Pouliot, T. Vogler, P. Renaud and A. Studer, Org. Lett., 2012, 14, 4474 CrossRef CAS PubMed; (e) S. E. Denmark and N. Carson, Org. Lett., 2015, 17, 5728 CrossRef CAS PubMed.
- For selected examples, see: (a) W. J. Close, J. Am. Chem. Soc., 1957, 79, 1455 CrossRef CAS; (b) T. Noguchi, M. Hasegawa, K. Tomisawa and M. Mitsukuchi, Bioorg. Med. Chem., 2003, 11, 4729 CrossRef CAS PubMed; (c) K. Lukin, M. C. Hsu, G. Chambournier, B. Kotecki, C. J. Venkatramani and M. R. Leanna, Org. Process Res. Dev., 2007, 11, 578 CrossRef CAS; (d) F. Bertolini, S. Crotti, V. D. Bussolo, F. Macchia and M. Pineschi, J. Org. Chem., 2008, 73, 8998 CrossRef CAS PubMed; (e) F. Yu, J.-N. Zhou, X.-C. Zhang, Y.-Z. Sui, F.-F. Wu, L.-J. Xie, A. S. C. Chan and J. Wu, Chem. – Eur. J., 2011, 17, 14234 CrossRef CAS PubMed; (f) O. Pablo, D. Guijarro and M. Yus, J. Org. Chem., 2013, 78, 9181 CrossRef CAS PubMed.
- For selected reviews, see: (a) R. H. Crabtree, Chem. Rev., 1985, 85, 245 CrossRef CAS; (b) A. K. Sadana, R. K. Saini and W. E. Billups, Chem. Rev., 2003, 103, 1539 CrossRef CAS PubMed; (c) C.-H. Jun, Chem. Soc. Rev., 2004, 33, 610 RSC; (d) T. Seiser and N. Cramer, Org. Biomol. Chem., 2009, 7, 2835 RSC; (e) T. Seiser, T. Saget, D. N. Tran and N. Cramer, Angew. Chem., Int. Ed., 2011, 50, 7740 CrossRef CAS PubMed; (f) A. Flores-Gaspar and R. Martin, Synthesis, 2013, 563 CAS; (g) F. Chen, T. Wang and N. Jiao, Chem. Rev., 2014, 114, 8613 CrossRef CAS PubMed; (h) L. Souillart, E. Parker and N. Cramer, Top. Curr. Chem., 2014, 346, 163 CrossRef CAS PubMed; (i) T. Xu, A. Dermenci and G. Dong, Top. Curr. Chem., 2014, 346, 233 CrossRef CAS PubMed; (j) A. Dermenci, J. W. Coe and G. Dong, Org. Chem. Front., 2014, 1, 567 RSC; (k) I. Marek, A. Masarwa, P. Delaye and M. Leibeling, Angew. Chem., Int. Ed., 2015, 54, 414 CAS; (l) L. Souillart and N. Cramer, Chem. Rev., 2015, 115, 9410 CrossRef CAS PubMed; (m) R. Ren and C. Zhu, Synlett, 2016, 1139 CAS; (n) H. Yan and C. Zhu, Prog. Chem., 2016, 28, 1 Search PubMed.
- For transition-metal catalyzed examples, see: (a) T. Nishimura, K. Ohe and S. Uemura, J. Am. Chem. Soc., 1999, 121, 2645 CrossRef CAS; (b) T. Nishimura and S. Uemura, J. Am. Chem. Soc., 1999, 121, 11010 CrossRef CAS; (c) T. Nishimura, S. Matsumura, Y. Maeda and S. Uemura, Chem. Commun., 2002, 50 RSC; (d) S. Matsumura, Y. Maeda, T. Nishimura and S. Uemura, J. Am. Chem. Soc., 2003, 125, 8862 CrossRef CAS PubMed; (e) T. Seiser and N. Cramer, J. Am. Chem. Soc., 2010, 132, 5340 CrossRef CAS PubMed; (f) A. Ziadi and R. Martin, Org. Lett., 2012, 14, 1266 CrossRef CAS PubMed; (g) A. Ziadi, A. Correa and R. Martin, Chem. Commun., 2013, 49, 4286 RSC; (h) N. Ishida, Y. Nakanishi and M. Murakami, Angew. Chem., Int. Ed., 2013, 52, 11875 CrossRef CAS PubMed; (i) J. Yu, H. Yan and C. Zhu, Angew. Chem., Int. Ed., 2016, 55, 1143 CrossRef CAS PubMed.
- For selected examples, see: (a) J. Rocek and A. E. Radkowsky, J. Am. Chem. Soc., 1968, 90, 2986 CrossRef CAS; (b) K. Meyer and J. Rocek, J. Am. Chem. Soc., 1972, 94, 1209 CrossRef CAS; (c) S. Tsunoi, I. Ryu, Y. Tamura, S. Yamasaki and N. Sonoda, Synlett, 1994, 1009 CrossRef CAS; (d) N. I. Kapustina, L. L. Sokova, V. D. Makhaev, L. A. Petrava and G. I. Nikishin, Russ. Chem. Bull., 1999, 48, 2080 CrossRef CAS; (e) B. M. Casey, C. A. Eakin and R. A. Flowers II, Tetrahedron Lett., 2009, 50, 1264 CrossRef CAS PubMed.
- (a) H. Zhao, X. Fan, J. Yu and C. Zhu, J. Am. Chem. Soc., 2015, 137, 3490 CrossRef CAS PubMed. For a highlight, see: (b) X. Fan, H. Zhao and C. Zhu, Acta Chim. Sin., 2015, 73, 979 CAS.
- R. Ren, H. Zhao, L. Huan and C. Zhu, Angew. Chem., Int. Ed., 2015, 54, 12692 CrossRef CAS PubMed.
- R. Ren, Z. Wu, Y. Xu and C. Zhu, Angew. Chem., Int. Ed., 2016, 55, 2866 CrossRef CAS PubMed.
- R. Ren, Z. Wu and C. Zhu, Chem. Commun., 2016, 52, 8160 RSC.
- D. Wang, R. Ren and C. Zhu, J. Org. Chem., 2016, 81, 8043 CrossRef CAS PubMed.
- X. Fan, H. Zhao, J. Yu, X. Bao and C. Zhu, Org. Chem. Front., 2016, 3, 227 RSC.
- F.-Q. Huang, J. Xie, J.-G. Sun, Y.-W. Wang, X. Dong, L.-W. Qi and B. Zhang, Org. Lett., 2016, 18, 684 CrossRef CAS PubMed.
- (a) P. R. Khoury, J. D. Goddard and W. Tam, Tetrahedron, 2004, 60, 8103 CrossRef CAS; (b) A. L. Ringer and D. H. Magers, J. Org. Chem., 2007, 72, 2533 CrossRef CAS PubMed.
- For selected examples: (a) A. Leyva-Pérez, J. R. Cabrero-Antonino, P. Rubio-Marqués, S. I. Al-Resayes and A. Corma, ACS Catal., 2014, 4, 722 CrossRef; (b) I. Salama, S. Löber, H. Hübner and P. Gmeiner, Bioorg. Med. Chem. Lett., 2014, 24, 3753 CrossRef CAS PubMed.
- For examples of generation of MnV by using the hypervalent iodine reagent, see: (a) W. Liu, X. Huang, M.-J. Cheng, R. J. Nielsen, W. A. Goddard III and J. T. Groves, Science, 2012, 337, 1322 CrossRef CAS PubMed; (b) W. Liu and J. T. Groves, Angew. Chem., Int. Ed., 2013, 52, 6024 CrossRef CAS PubMed; (c) X. Huang, W. Liu, H. Ren, R. Neelamegam, J. M. Hooker and J. T. Groves, J. Am. Chem. Soc., 2014, 136, 6842 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00443a |
| This journal is © the Partner Organisations 2016 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PIDA (0.4 mmol, 2.0 equiv.) was used.
PIDA (0.4 mmol, 2.0 equiv.) was used.