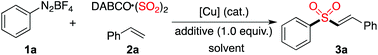A copper(II)-catalyzed three-component reaction of aryldiazonium tetrafluoroborates, sulfur dioxide, with alkenes†
Runyu
Mao
ab,
Zheng
Yuan
b,
Rui
Zhang
b,
Yechun
Ding
a,
Xiaona
Fan
*a and
Jie
Wu
*bc
aGannan Medical University Collaborative Innovation Center for Gannan Oil-tea Camellia Industrial Development, 1 Yixueyuan Road, Ganzhou, Jiangxi 341000, China. E-mail: fxn918@126.com
bDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 7th September 2016
Abstract
A three-component reaction of aryldiazonium tetrafluoroborates, sulfur dioxide, with alkenes catalyzed by copper(II) bromide is developed, which provides an efficient route to (E)-alkenyl sulfones or allylic sulfones via the insertion of sulfur dioxide. A radical process is believed to be involved in the reaction.
In the past few years, progress in the synthesis of sulfonyl compounds via insertion of sulfur dioxide has been achieved rapidly.1–3 Usually, the easily handled DABCO-(SO2)2 and inorganic sulfites were used as the source of sulfur dioxide in organic transformations, instead of toxic gaseous sulfur dioxide. It is well known that sulfonyl compounds are largely present in pharmaceutical and agrochemical molecules.4,5 Consistent with our continuing interest in the development of methods and construction of libraries of natural product-like compounds,6 we are also interested in the generation of bioactive compounds with the sulfonyl unit. Recently, we reported several routes for the preparation of sulfonyl compounds via the insertion of sulfur dioxide through a radical process.3 For instance, aryl N-aminosulfonamides could be efficiently synthesized via a coupling of aryldiazonium tetrafluoroborates, DABCO·(SO2)2 and hydrazines under metal-free conditions.3a This reaction was demonstrated to proceed through a radical process. Further exploration of this work revealed that the aminosulfonylation through insertion of sulfur dioxide could be extended to aryl/alkyl halides enabled by photoenergy.3b Due to the importance of vinyl sulfones in natural products and biologically active molecules,7 as well as in organic synthesis,8 we envisioned that the radical process of fixation of sulfur dioxide might be applied to the synthesis of vinyl sulfones.
So far, many approaches have been reported for the preparation of vinyl sulfones.9,10 For example, Tiwari and co-workers reported the stereoselective synthesis of E-vinyl sulfones catalyzed by Cu0/Fe3O4 from tosylmethyl isocyanide and alkynes.10h Among the methods developed, using sulfur dioxide as the source of sulfonyl groups would be an ideal route. In 2015, Feng and co-workers reported the synthesis of vinyl sulfones via iodide-catalyzed radical alkenylation of aryl diazonium salts, terminal alkenes and DABCO·(SO2)2 in the presence of an oxidant (TBHP).2q However, we found that some results could not be reproducible. For instance, to our surprise, only a trace amount of the product of (E)-(2-(phenylsulfonyl)vinyl)benzene was detected for the reaction of phenyldiazonium tetrafluoroborate, sulfur dioxide, with styrene under the reported conditions. Additionally, ethyldiazonium tetrafluoroborate which is extremely unstable and cannot be accessible, was applied successfully in the reaction, as described by Feng.2q Prompted by the importance of vinyl sulfones and encouraged by our previous work for the insertion of sulfur dioxide with aryldiazonium tetrafluoroborates, we wished to explore the reaction of aryldiazonium tetrafluoroborates, sulfur dioxide, with alkenes. Herein, we report our recent efforts for the generation of vinyl sulfones through a copper(II)-catalyzed reaction of aryldiazonium tetrafluoroborates, sulfur dioxide, and alkenes. In some cases, allylic sulfones were obtained under the conditions.
Initially, a three-component reaction of phenyldiazonium tetrafluoroborate 1a, sulfur dioxide, with styrene 2a was selected as the model for reaction development. Since the presence of a copper catalyst would enable aryldiazonium tetrafluoroborate to produce the aryl radical, at the outset, the reaction was performed in the presence of copper(I) bromide (10 mol%) in MeCN at 80 °C (Table 1, entry 1). To our delight, the desired (E)-alkenyl sulfone 3a was isolated in 38% yield. A slightly lower yield (33%) was observed when the solvent was changed to DMF (Table 1, entry 2). No better results were obtained when the reaction was performed in DMSO, toluene, 1,4-dioxane, or DCE (Table 1, entries 3–6). A similar yield was generated when the reaction took place at 100 °C (Table 1, entry 7). However, an inferior yield was isolated when the reaction temperature was changed to 60 °C (Table 1, entry 8). Interestingly, the yield was increased to 46% when the amount of copper(I) bromide was changed to 20 mol% (Table 1, entry 9). Other copper catalysts were examined subsequently (Table 1, entries 10–16). Since Cu(II) could be converted to Cu(I) in the presence of sulfur dioxide,11 several copper(II) salts were evaluated as well. It was found that the reaction proceeded efficiently when copper(II) bromide was employed, giving rise to the corresponding product 3a in 50% yield (Table 1, entry 14). An improved yield was observed when 2.5 equivalents of DABCO·(SO2)2 were used in the reaction (Table 1, entry 17). We conceived that the presence of an acid would promote the release of sulfur dioxide from the complex of DABCO·(SO2)2. Therefore, further screening of acids as additives was performed, which revealed that the yield could be dramatically improved (Table 1, entries 18–20). Gratifyingly, the reaction proceeded smoothly in the presence of acetic acid, leading to the expected product 3a in 90% yield (Table 1, entry 18). The yield was lower when the amount of acetic acid was reduced to 0.5 equivalents (Table 1, entry 21).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | [Cu] | Additive | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: phenyldiazonium tetrafluoroborate 1a (0.2 mmol), DABCO·(SO2)2 (0.25 mmol), styrene 2a (0.4 mmol), copper catalyst (10 mol%), additive (1.0 equiv.), solvent (1.0 mL), under N2. b Isolated yield based on phenyldiazonium tetrafluoroborate 1a. c In the presence of 20 mol% copper catalyst. d In the presence of 2.5 equivalents of DABCO·(SO2)2. e In the presence of 0.5 equivalents of HOAc. | |||||
| 1 | CuBr | MeCN | 80 | 38 | |
| 2 | CuBr | DMF | 80 | 33 | |
| 3 | CuBr | DMSO | 80 | 25 | |
| 4 | CuBr | Toluene | 80 | Trace | |
| 5 | CuBr | Dioxane | 80 | Trace | |
| 6 | CuBr | DCE | 80 | <10 | |
| 7 | CuBr | MeCN | 100 | 37 | |
| 8 | CuBr | MeCN | 60 | 29 | |
| 9c | CuBr | MeCN | 80 | 46 | |
| 10c | CuCl | MeCN | 80 | 10 | |
| 11c | CuI | MeCN | 80 | 21 | |
| 12c | CuOAc | MeCN | 80 | <10 | |
| 13c | Cu2O | MeCN | 80 | <10 | |
| 14c | CuBr2 | MeCN | 80 | 50 | |
| 15c | CuCl2 | MeCN | 80 | 19 | |
| 16c | Cu(OAc)2 | MeCN | 80 | <10 | |
| 17c,d | CuBr2 | MeCN | 80 | 63 | |
| 18c,d | CuBr2 | HOAc | MeCN | 80 | 90 |
| 19c,d | CuBr2 | PhCO2H | MeCN | 80 | 82 |
| 20c,d | CuBr2 | TFA | MeCN | 80 | 63 |
| 21c,d,e | CuBr2 | HOAc | MeCN | 80 | 72 |
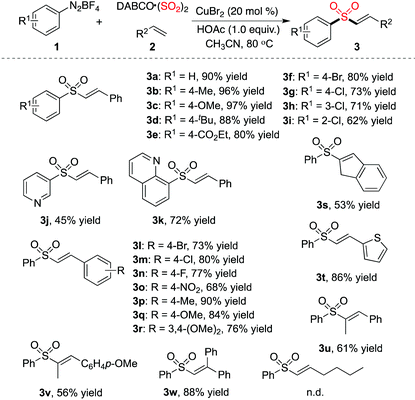
Since the optimized conditions shown in Table 1 were effective for the generation of (E)-alkenyl sulfones 3, we thus started to explore the scope of the three-component reaction of aryldiazonium tetrafluoroborates 1, sulfur dioxide, with alkenes 2 catalyzed by copper(II) bromide. The result is shown in Table 2. It was found that various aryldiazonium tetrafluoroborates 1 coupled well in the reactions of sulfur dioxide and styrene 2a, providing the corresponding (E)-alkenyl sulfones 3 in good to excellent yields. A wide range of functional groups was found to be compatible under the standard reaction conditions, including methoxy, chloride, bromide, and ester groups. For instance, ester-substituted sulfone 3e was obtained in 80% yield. It is noteworthy that the reactions of (hetero)aryldiazonium tetrafluoroborates 1 with sulfur dioxide and styrene 2a proceeded efficiently as well, leading to the desired products (compounds 3j and 3k). Different alkenes were examined subsequently in the reaction of phenyldiazonium tetrafluoroborate 1a and sulfur dioxide. Electron-withdrawing and electron-donating groups attached on the aromatic ring of styrenes were all tolerated under the conditions. 2-Vinylthiophene was a good coupling partner as well in the reaction, which afforded the corresponding product 3t in 86% yield. Reactions of 1,2-disubstituted olefins were explored in the meantime. As expected, the desired products were generated in moderate yields (compounds 3u and 3v). Ethene-1,1-diyldibenzene reacted with phenyldiazonium tetrafluoroborate 1a and sulfur dioxide, providing the expected product 3w in 88% yield. However, no reaction occurred when 1-hexene was employed as a replacement.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| a Isolated yield based on aryldiazonium tetrafluoroborate 1. |
|---|
|
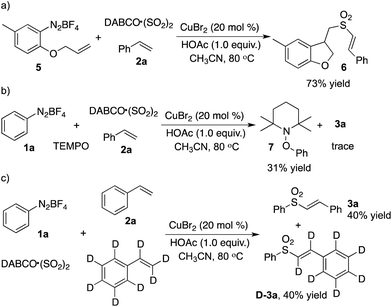
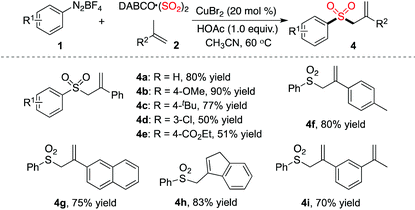
Interestingly, different outcomes were obtained when prop-1-en-2-ylbenzene was used as a substrate in the reaction of phenyldiazonium tetrafluoroborate 1a with sulfur dioxide. Allylic sulfones were formed instead of (E)-alkenyl sulfones. The result is summarized in Table 3. Optimization of preliminary conditions revealed that the reaction proceeded efficiently at 60 °C, leading to the corresponding allylic sulfone 4a in 80% yield. Again, the substitutions attached onto the aromatic ring of aryldiazonium tetrafluoroborate 1 were well tolerated under the conditions. For example, the ester group remained intact during the reaction process, which provided the corresponding product 4e in 51% yield. Reactions of other alkenes with phenyldiazonium tetrafluoroborate 1a and sulfur dioxide were investigated subsequently. As shown in Table 3, all reactions proceeded smoothly to afford the corresponding products in good yields.
| a Isolated yield based on aryldiazonium tetrafluoroborate 1. |
|---|
|
Based on our previous reports,3a we postulated that this reaction might proceed through a radical process. Thus, the following experiments were designed and performed (Scheme 1). For the three-component reaction of compound 5, sulfur dioxide and styrene, we observed the formation of the desired product 6 (Scheme 1, eqn (a)). The result indicated that a radical tandem process might be involved. Addition of TEMPO into the reaction of phenyldiazonium tetrafluoroborate 1a, sulfur dioxide and styrene 2a afforded compound 7 (Scheme 1, eqn (b)), which confirmed our hypothesis. The further deuterium experiment revealed that the formation of a double bond was not the rate-determining step (Scheme 1, eqn (c)).
A plausible mechanism for the copper(II)-catalyzed three-component reaction of aryldiazonium tetrafluoroborates, sulfur dioxide, with alkenes was then proposed (Scheme 2). We reasoned that Cu(II) would be converted to Cu(I) in the presence of sulfur dioxide,11 which would promote the formation of aryl radical A. The subsequent insertion of sulfur dioxide would occur to produce radical B, which would then undergo addition to the double bond of alkene 2 to afford radical C. Followed by electron transfer would lead to cation D, which would go through deprotonation to furnish the corresponding product 3.
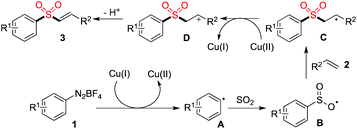 | ||
| Scheme 2 A plausible mechanism for the copper(II)-catalyzed three-component reaction of aryldiazonium tetrafluoroborates, sulfur dioxide, with alkenes. | ||
In conclusion, we have reported a three-component reaction of aryldiazonium tetrafluoroborates, sulfur dioxide, with alkenes catalyzed by copper(II) bromide. This transformation provides an efficient route to (E)-alkenyl sulfones or allylic sulfones via the insertion of sulfur dioxide. A radical process is believed to be involved in the reaction. This approach is attractive, and the corresponding (E)-alkenyl sulfones or allylic sulfones can be obtained in moderate to good yields.
Acknowledgements
Financial support from the National Natural Science Foundation of China (no. 21372046, 21532001) and the Opening Project of Gannan Medical University Collaborative Innovation Center for Gannan Oil-tea Camellia Industrial Development is gratefully acknowledged.Notes and references
- For reviews: (a) G. Liu, C. Fan and J. Wu, Org. Biomol. Chem., 2015, 13, 1592 RSC; (b) P. Bisseret and N. Blanchard, Org. Biomol. Chem., 2013, 11, 5393 RSC; (c) A. S. Deeming, E. J. Emmett, C. S. Richards-Taylor and M. C. Willis, Synthesis, 2014, 2701 Search PubMed. For examples, see: (d) D. Zheng, R. Mao, Z. Li and J. Wu, Org. Chem. Front., 2016, 3, 359 RSC; (e) S. Ye and J. Wu, Chem. Commun., 2012, 48, 7753 RSC; (f) S. Ye and J. Wu, Chem. Commun., 2012, 48, 10037 RSC; (g) S. Ye, H. Wang, Q. Xiao, Q. Ding and J. Wu, Adv. Synth. Catal., 2014, 356, 3225 CrossRef CAS; (h) Y. Luo, X. Pan, C. Chen, L. Yao and J. Wu, Chem. Commun., 2015, 51, 180 RSC; (i) A. S. Tsai, J. M. Curto, B. N. Rocke, A. R. Dechert-Schmitt, G. K. Ingle and V. Mascitti, Org. Lett., 2016, 18, 508 CrossRef CAS PubMed; (j) R. Mao, D. Zheng, H. Xia and J. Wu, Org. Chem. Front., 2016, 3, 693 RSC; (k) D. Zheng, M. Chen, L. Yao and J. Wu, Org. Chem. Front., 2016, 3, 985 RSC; (l) D. Zheng, Y. Kuang and J. Wu, Org. Biomol. Chem., 2015, 13, 10370 RSC; (m) W. Zhang and M. Luo, Chem. Commun., 2016, 52, 2980 RSC.
- (a) B. Nguyen, E. J. Emmet and M. C. Willis, J. Am. Chem. Soc., 2010, 132, 16372 CrossRef CAS PubMed; (b) E. J. Emmet, C. S. Garcia-Rubia, B. Nguyen, A. B. Garcia-Rubia, R. Hayter and M. C. Willis, Org. Biomol. Chem., 2012, 10, 4007 RSC; (c) L. Martial, Synlett, 2013, 1595 Search PubMed; (d) W. Li, H. Li, P. Langer, M. Beller and X.-F. Wu, Eur. J. Org. Chem., 2014, 3101 CrossRef; (e) W. Li, M. Beller and X.-F. Wu, Chem. Commun., 2014, 50, 9513 RSC; (f) X. Wang, L. Xue and Z. Wang, Org. Lett., 2014, 16, 4056 CrossRef CAS PubMed; (g) A. S. Deeming, C. J. Russell and M. C. Willis, Angew. Chem., Int. Ed., 2015, 54, 1168 CrossRef CAS PubMed; (h) C. C. Chen and J. Waser, Org. Lett., 2015, 17, 736 CrossRef CAS PubMed; (i) A. S. Deeming, C. J. Rusell, A. J. Hennessy and M. C. Willis, Org. Lett., 2014, 16, 150 CrossRef CAS PubMed; (j) B. N. Rocke, K. B. Bahnck, M. Herr, S. Lavergne, V. Mascitti, C. Perreault, J. Polivkova and A. Shavnya, Org. Lett., 2014, 16, 154 CrossRef CAS PubMed; (k) E. J. Emmett, B. R. Hayter and M. C. Willis, Angew. Chem., Int. Ed., 2013, 52, 12679 CrossRef CAS PubMed; (l) E. J. Emmett, B. R. Hayter and M. C. Willis, Angew. Chem., Int. Ed., 2014, 53, 10204 CrossRef CAS PubMed; (m) A. Shavnya, S. B. Coffey, A. C. Smith and V. Mascitti, Org. Lett., 2013, 15, 6226 CrossRef CAS PubMed; (n) M. W. Johnson, S. W. Bagley, N. P. Mankad, R. G. Bergman, V. Mascitti and F. D. Toste, Angew. Chem., Int. Ed., 2014, 53, 4404 CrossRef CAS PubMed; (o) A. Shavnya, K. D. Hesp, V. Mascitti and A. C. Smith, Angew. Chem., Int. Ed., 2015, 54, 13571 CrossRef CAS PubMed; (p) A. S. Deeming, C. J. Russell and M. C. Willis, Angew. Chem., Int. Ed., 2016, 55, 747 CrossRef CAS PubMed; (q) W. Fan, J. Su, D. Shi and B. Feng, Tetrahedron, 2015, 71, 6740 CrossRef CAS.
- (a) D. Zheng, Y. An, Z. Li and J. Wu, Angew. Chem., Int. Ed., 2014, 53, 2451 CrossRef CAS PubMed; (b) Y. Li, D. Zheng, Z. Li and J. Wu, Org. Chem. Front., 2016, 3, 574 RSC; (c) Y. An, D. Zheng and J. Wu, Chem. Commun., 2014, 50, 11746 RSC; (d) D. Zheng, Y. Li, Y. An and J. Wu, Chem. Commun., 2014, 50, 8886 RSC; (e) K. Zhou, H. Xia and J. Wu, Org. Chem. Front., 2016, 3, 865 RSC; (f) D. Zheng, J. Yu and J. Wu, Angew. Chem., Int. Ed., 2016, 55 DOI:10.1002/anie.201607292.
- (a) M. Bartholow, Top 200 Drugs of 2011. Pharmacy Times. http://www.pharmacytimes.com/publications/issue/2012/July2012/Top-200-Drugs-of-2011, accessed on Jan 9, 2013; (b) For a list of topdrugs by year, see: http://cbc.arizona.edu/njardarson/group/top-pharmaceuticals-poster, accessed on Jan 9, 2013; (c) J. Drews, Science, 2000, 287, 1960 CrossRef CAS PubMed.
- (a) P. J. Crowley, J. Fawcett, B. M. Kariuki, A. C. Moralee, J. M. Percy and V. Salafia, Org. Lett., 2002, 4, 4125 CrossRef CAS PubMed; (b) N. S. Simpkins, Sulfones in Organic Synthesis, Pergamon Press, Oxford, 1993 Search PubMed.
- For selected reviews and accounts, see: (a) G. Qiu and J. Wu, Chem. Rec., 2016, 16, 19 CrossRef CAS PubMed; (b) Y. Luo, X. Pan, X. Yu and J. Wu, Chem. Soc. Rev., 2014, 43, 834 RSC; (c) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (d) G. Qiu, Y. Kuang and J. Wu, Adv. Synth. Catal., 2014, 356, 3483 CrossRef CAS; (e) H. Wang, Y. Kuang and J. Wu, Asian J. Org. Chem., 2012, 1, 302 CrossRef CAS; (f) L. He, H. Nie, G. Qiu, Y. Gao and J. Wu, Org. Biomol. Chem., 2014, 12, 9045 RSC; (g) G. Qiu and J. Wu, Synlett, 2014, 2703 CAS.
- For selected examples, see: (a) U. Mahesh, K. Liu, R. C. Panicker and S. Q. Yao, Chem. Commun., 2007, 1518 Search PubMed; (b) G. Wang, U. Mahesh, G. Y. J. Chen and S. Yao, Org. Lett., 2003, 5, 737 CrossRef CAS PubMed; (c) I. Forristal, J. Sulfur Chem., 2005, 26, 163 CrossRef CAS; (d) F. Hof, A. Schutz, C. Fah, S. Meyer, D. Bur, J. Liu, D. E. Goldberg and E. Diederich, Angew. Chem., Int. Ed., 2006, 45, 2138 CrossRef CAS PubMed; (e) L. Ni, X. Zheng, P. K. Somers, L. K. Hoong, R. R. Hill, E. M. Marino, K. L. Suen, U. Saxena and C. Meng, Bioorg. Med. Chem. Lett., 2003, 13, 745 CrossRef CAS PubMed; (f) J. T. Palmer, D. Rasnick, J. L. Klaus and D. Brcmme, J. Med. Chem., 1995, 38, 3193 CrossRef CAS PubMed; (g) M. M. M. Santos and R. Moreira, Mini-Rev. Med. Chem., 2007, 7, 1040 CrossRef CAS PubMed; (h) R. Ettari, E. Nizi, M. E. D. Francesco, M. A. Dude, G. Pradel, R. Vicik, T. Schirmeister, N. Micale, S. Grasso and M. Zappala, J. Med. Chem., 2008, 51, 988 CrossRef CAS PubMed.
- For selected examples, see: (a) M. N. Noshi, A. El-Awa, E. Torres and P. L. Fuchs, J. Am. Chem. Soc., 2007, 129, 11242 CrossRef CAS PubMed; (b) J. N. Desrosiers and A. B. Charette, Angew. Chem., Int. Ed., 2007, 46, 5955 CrossRef CAS PubMed; (c) G. Pandey, K. N. Tiwari and V. G. Puranik, Org. Lett., 2008, 10, 3611 CrossRef CAS PubMed; (d) K. Oh, Org. Lett., 2007, 9, 2973 CrossRef CAS PubMed.
- For selected examples, see: (a) S. Choi, J. Yang, M. Ji, H. Choi, M. Kee, K. Ahn, S. Byeon, W. Baik and S. Koo, J. Org. Chem., 2001, 66, 8192 CrossRef CAS PubMed; (b) A. A. Linden, L. Kruger and J. Backvall, J. Org. Chem., 2003, 68, 5890 CrossRef CAS PubMed; (c) E. Baciocchi, M. F. Gerini and A. Lapi, J. Org. Chem., 2004, 69, 3586 CrossRef CAS PubMed; (d) F. G. Gelalcha and B. Schulze, J. Org. Chem., 2002, 67, 8400 CrossRef CAS PubMed; (e) M. Matteucci, G. Bhalay and M. Bradley, Org. Lett., 2003, 5, 235 CrossRef CAS PubMed; (f) S. Chodroff and W. F. Whitmore, J. Am. Chem. Soc., 1950, 72, 1073 CrossRef CAS; (g) P. B. Hopkins and P. L. Fuchs, J. Org. Chem., 1978, 43, 1208 CrossRef CAS; (h) R. A. Gancarz and J. L. Kice, Tetrahedron Lett., 1980, 21, 4155 CrossRef CAS; (i) J. M. Baskin and Z. Wang, Org. Lett., 2002, 4, 4423 CrossRef CAS PubMed; (j) W. Zhu and D. Ma, J. Org. Chem., 2005, 70, 2696 CrossRef CAS PubMed; (k) M. Bian, F. Xu and C. Ma, Synthesis, 2007, 2951 CAS; (l) D. C. Reeves, S. Rodriguez, H. Lee, N. Hahhad, D. Krishnamurthy and C. H. Senanayake, Tetrahedron Lett., 2009, 50, 2870 CrossRef CAS; (m) S. Cacchi, G. Fabrizi, A. Goggiamani, L. M. Parisi and R. Bernini, J. Org. Chem., 2004, 69, 5608 CrossRef CAS PubMed; (n) J. Sinnreich and M. Asscher, J. Chem. Soc., Perkin Trans. 1, 1972, 1543 RSC; (o) W. E. Truce and C. T. Goralski, J. Org. Chem., 1971, 36, 2536 CrossRef CAS; (p) N. Taniguchi, Synlett, 2011, 1308 CrossRef CAS.
- For selected examples, see: (a) F. Huang and R. A. Batey, Tetrahedron, 2007, 63, 7667 CrossRef CAS; (b) V. Nair, A. Augustine, T. G. George and L. G. Nair, Tetrahedron Lett., 2001, 42, 6763 CrossRef CAS; (c) J. Chen, J. Mao, Y. Zheng, D. Liu, G. Rong, H. Yan, C. Zhang and D. Shi, Tetrahedron, 2015, 71, 5059 CrossRef CAS; (d) Y. Xu, J. Zhao, X. Tang, W. Wu and H. Jiang, Adv. Synth. Catal., 2014, 356, 2029 CrossRef CAS; (e) S. Tang, Y. Wu, W. Liao, R. Bai, C. Liu and A. Lei, Chem. Commun., 2014, 50, 4496 RSC; (f) P. Katrun, S. Chiampanichayakul, K. Korworapan, M. Pohmakotr, V. Reutrakul, T. Jaipetch and C. Kuhakarn, Eur. J. Org. Chem., 2010, 5633 CrossRef CAS; (g) R. Song, Y. Liu, Y. Liu and J. Li, J. Org. Chem., 2011, 76, 1001 CrossRef CAS PubMed; (h) M. Phanindrudu, D. K. Tiwari, B. Sridhar, P. R. Likhar and D. K. Tiwari, Org. Chem. Front., 2016, 3, 795 RSC.
- H. Meerwein, G. Dittmar, R. Gollner, K. Hafner, F. Mensch and O. Steinfort, Chem. Ber., 1957, 90, 841 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00350h |
| This journal is © the Partner Organisations 2016 |