The effect of backbone flexibility on site-selective modification of macrocycles†
Abstract
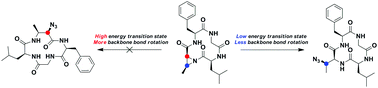
As the emerging modality in drug discovery, macrocycles represent topical targets of chemical synthesis. However, the influence of conformational effects on their reactivity has not received enough attention. We demonstrate that the regiochemistry of nucleophilic attack on aziridine-containing macrocyclic systems varies according to macrocycle ring size and that the influence of remote torsional interactions in the macrocycle ultimately determines the regioselectivity of the reactions. These findings should facilitate the development of divergent strategies of macrocycle synthesis using post-cyclization modification.

 Please wait while we load your content...
Please wait while we load your content...