Plant-derived nanostructures: types and applications
Reza
Mohammadinejad
a,
Samaneh
Karimi
b,
Siavash
Iravani
*c and
Rajender S.
Varma
*d
aYoung Researchers and Elite Club, Sirjan Branch, Islamic Azad University, Sirjan, Iran
bThe New Zealand Institute for Plant & Food Research, 7608 Lincoln, New Zealand
cBiotechnology Department, Faculty of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran. E-mail: siavashira@gmail.com
dSustainable Technology Division, National Risk Management Research Laboratory, US Environmental Protection Agency, MS 443, 26 West Martin Luther King Drive, Cincinnati, Ohio 45268, USA. E-mail: Varma.Rajender@epa.gov
First published on 6th October 2015
Abstract
Plant-derived nanostructures and nanoparticles (NPs) have functional applications in numerous disciplines such as health care, food and feed, cosmetics, biomedical science, energy science, drug-gene delivery, environmental health, and so on. Consequently, it is imperative for researchers to understand that plants are cost-effective, sustainable and renewable platforms, and therefore, they are ideal sources for production of natural NPs. This critical review discusses significant recent developments pertaining to plant-derived nanostructures, their classes, and vital applications. The aim is to provide insight into the use of plants as bio-renewable, sustainable, diversified resources and as platforms for the production of useful nanostructures and NPs, with functions in various fields including medicine, industry, agriculture, and pharmaceuticals.
1. Introduction
Nanostructures and nanoparticles (NPs) have been extensively studied owing to their extremely diminutive size and large surface-to-volume ratio, which contribute to both physical and chemical differences in their properties (e.g., mechanical, biological and sterical properties, catalytic activity, thermal and electrical conductivity, optical absorption, and melting point), when compared to bulk materials of an identical chemical composition. These nanostructures and NPs have been used in areas such as mechanics, optics, biomedical sciences, chemicals, electronics, space industries, drug-gene delivery, energy science, catalysis,1,2 optoelectronic devices,3,4 photo-electrochemical applications,5 and nonlinear optical devices.6,7 For instance, biocompatible macromolecules such as plant proteins can be used as drug carriers. Azmi et al.8 reported the use of ultra-sensitive, silicon nanowire-based biosensor devices for the detection of 8-hydroxydeoxyguanosine (8-OHdG), a biomarker for prostate cancer risk. The speed, sensitivity, and ease of biomarker detection using these ultra-sensitive biosensors render them ideal for eventual point-of-care diagnostics. Moreover, a range of nanometer-sized materials, including metal and metal oxide NPs, semiconductor quantum dots, carbon nanomaterials and polymer NPs have been used for various important pharmaceutical and medical applications (for example, nano polymerase chain reaction, nano-PCR). This strategy afforded a potentially powerful PCR technology, with unprecedented sensitivity, selectivity, and extension rates.9,10Nanomaterials are generally prepared by a variety of mechanical, physical, and chemical approaches. Organisms (microorganisms and plants) possess unique properties which offer many advantages, including widespread availability, sustainability, and inherent inclusion of chemical functionality, biocompatibility, and biodegradability.11 Researchers have found inspiration from viruses, bacteria, fungi, algae, and biomolecules to produce biomimetic nanostructures for various applications. For example, the application of plant virus-derived nanostructures in materials science, biomedical research, and engineering has been recently advanced by the development of fluorescence-labeled viruses for optical imaging in tissue culture and pre-clinical animal models. Most studies have focused their attention on the applications of viruses chemically modified with organic dyes. Shukla et al.12 studied genetically-engineered virus-based biomaterials that incorporated green or red fluorescent proteins, and reported that the genetic introduction of imaging moieties was advantageous because post-harvest modification was not required, thus minimizing the number of manufacturing steps needed and maximizing the yield of each fluorescent probe.
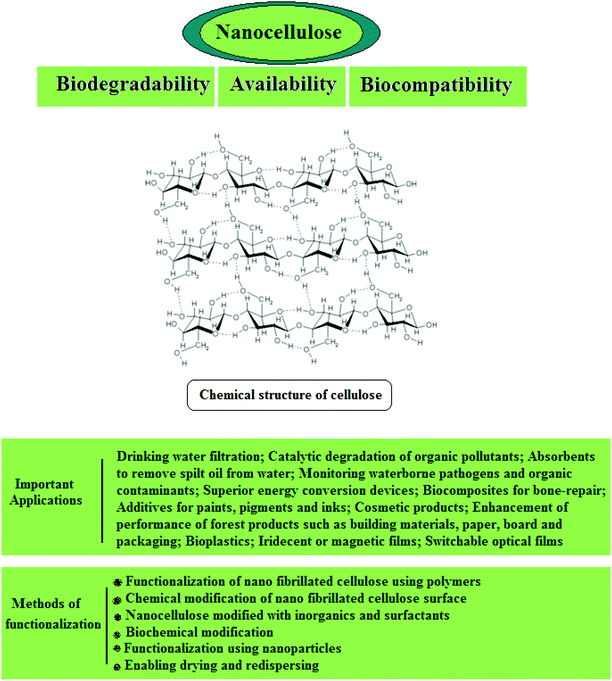
Plants have the ability of producing a variety of highly ordered hierarchical structures, and they can generate such structures effortlessly in various sizes. They present a range of constructions which comprise the natural fractal geometry of branches to form barbed raphide crystals and thin stacks of grana thylakoids in chloroplasts. Actually, organisms can produce a vast variety of sophisticated inorganic materials. Biomolecules offer unique functionalities such as specific recognition capabilities or catalytic activity. Biological subunits, based on these recognition capabilities, can self-assemble into defined superstructures with unique shapes. Moreover, they respond to multiple physical, chemical, or biological stimuli, and consequently provide a potential means for manufacturing nano-machines.13 There is typically a high level of intricacy in natural plant structures at the micrometer and nanometer scales which is a representation of the sophistication of current engineered materials and systems. There are various synthetic methods to transform highly structured plant materials into functional materials so as to exploit their intrinsic natural morphology. For instance, it is becoming common to utilize new hybrid structures for drug delivery, environmental remediation, energy generation, or bio-templated nanofabrication. Furthermore, a higher biodegradability trait is generated as a consequence of the direct incorporation of plant structures within polymeric and carbonaceous materials; this bodes a high level of promise for plant composites in biomedical and biosensing applications.14–20 Plants are capable of generating a wide variety of advanced nanostructures matching the sophistication of current engineered materials, wherein plant biomolecules mediate the safest and most cost-effective large-scale production of biocompatible NPs. Zein, starch, and ivy NPs are just a few examples of biomolecules which have broad important applications in various pharmaceutical and medical fields. This review begins with a description of plant-derived nanostructures and NPs as building blocks for nanotechnology and their important applications. The advantages of using bio-renewable and bio-degradable plants and agricultural residues as sources for nanostructure production are also highlighted (Fig. 1). An integral portion of this article focuses on plant-derived NPs and their significance, and a brief discussion on the important role of plants in nanoparticle synthesis is provided. Moreover, the diverse applications of plant-derived nanostructures and NPs in biology, medicine, and pharmacy are discussed in this review.
 | ||
| Fig. 1 Salient advantages of using plants and agricultural residues as resources for nanostructure production. | ||
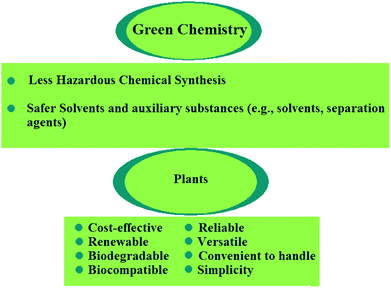
2. Plants and green nanotechnology
In green nanotechnology, plants have been used owing to their non-toxic chemical constituents and natural materials. Green nanotechnology helps to eliminate or minimize harmful polluting substances in the synthesis of nanomaterials (Fig. 2). There are nearly 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plant species in the world which convert sunlight energy into organic chemical energy, in the form of carbohydrates, via photosynthesis.21 Bioactive and biodegradable compounds obtained from medicinal herbs are very effective for the treatment of several human diseases. For instance, taxol (an approved anticancer drug) and silymarin have been identified as the most effective therapies for liver diseases. Plants are the source of raw materials, such as wood, resins, oils, and dyes for agro-based industries. Production of biofuels from plants (e.g., sugarcane and jatropha) has become a potential alternative for fossil fuels. Apart from their commercial value, plants are advantageous because they clean the atmosphere, water, and soil via uptake of large amounts of CO2, heavy metals, and other pollutants. In addition, the significant potential for plant nanostructures has been realized.
000 plant species in the world which convert sunlight energy into organic chemical energy, in the form of carbohydrates, via photosynthesis.21 Bioactive and biodegradable compounds obtained from medicinal herbs are very effective for the treatment of several human diseases. For instance, taxol (an approved anticancer drug) and silymarin have been identified as the most effective therapies for liver diseases. Plants are the source of raw materials, such as wood, resins, oils, and dyes for agro-based industries. Production of biofuels from plants (e.g., sugarcane and jatropha) has become a potential alternative for fossil fuels. Apart from their commercial value, plants are advantageous because they clean the atmosphere, water, and soil via uptake of large amounts of CO2, heavy metals, and other pollutants. In addition, the significant potential for plant nanostructures has been realized.
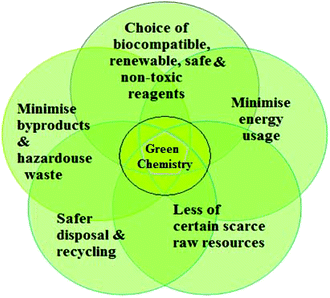 | ||
| Fig. 2 Principles of green chemistry for the production of safer and more sustainable nanomaterials via sustainable nano-manufacturing processes. | ||
2.1. Role of plants and phytochemicals in nanoparticle synthesis
Phytochemicals and plant extracts can be used as reducing and stabilizing agents for synthesis of metal and metal oxide NPs in a single-step green process (Table 1). The only way to develop these “green” processes is to adapt benign synthesis approaches that use mild reaction conditions and non-toxic reaction precursors.22–33 This biogenic reduction of metal ions to metallic NPs is inexpensive, non-toxic, eco-friendly, expeditious, and is readily accomplished at ambient temperature and pressure with great potential for scaling up. Synthesis mediated by plant extracts is an environmentally friendly option as the reducing agents involved encompass various water-soluble plant metabolites (e.g., alkaloids, phenolic compounds, quinones, organic acids, flavonoids, terpenoids, and catechins), and co-enzymes. Extracts from a diverse range of plant species are efficaciously used for the manufacture of metal and metal oxide NPs. In addition to plant extracts, live plants can also be used for nanoparticle synthesis.34–36 Green synthesis of metal and metal oxide NPs by plants is mainly advantageous in terms of environmental friendliness. But, the major drawbacks associated with this green process are longer reaction times, tedious purification steps, and greater sizes of all NPs and poor understanding of the underlying mechanisms. Optimization of these green chemistry methods is critical for fast and clean synthesis of NPs with the desired sizes and morphologies. Plant-mediated synthesis of NPs is prefered due to the presence of phytochemicals and biomolecules such as proteins, amino acids, vitamins, polysaccharides, polyphenols, terpenoids, and organic acids. These molecules can be used in synthesis, and also in the stabilization of metal and metal oxide NPs formed with the desired sizes and shapes. Studies have indicated that these molecules not only play a role in reducing the ions to nanosize, but also play an important role in the capping of NPs.20,23,32,34,37–48| Plants | Nanoparticles | Phytochemicals/biomolecules | Ref. |
|---|---|---|---|
| Allium sativum | Au | Proteins | 49 |
| Aloe barbadensis | CuO | Phenolic compounds, terpenoids, proteins | 50 |
| Alternanthera sessilis Linn. | Ag | Alkaloids, tannins, ascorbic acid, carbohydrates, proteins | 51 |
| Andrographis paniculata Nees. | Ag | Hydroxyflavones, catechins | 52 |
| Annona squamosa | Pd | Secondary metabolites contained –OH group | 53 |
| Astragalus gummifer Labill. | Ag | Proteins | 54 |
| Azadirachta indica | Ag | Reducing sugars, terpenoids | 55 |
| Azadirachta indica A. Juss. | Au | Salanin, nimbin, azadirone, azadirachtins | 56 |
| Benincasa hispida | Au | Polyols | 57 |
| Calotropis procera L. | Cu | Cysteine protease and tryptophan with functional groups of amines, alcohols, ketones, aldehydes, and carboxylic acids | 58 |
| Camellia sinensis | Au | Polyphenolic compounds | 59 |
| Carica papaya (fruit extract) | Ag | Hydroxyflavones, catechins | 60 |
| Carica papaya (callus extract) | Ag | Proteins and other ligands | 61 |
| Cinnamomum zeylanicum | Pd | Terpenoids (e.g., linalool, methyl chavicol, and eugenol) | 62, 63 |
| Cinnamomum camphora | Pd | Polyol components, water soluble heterocyclic components | 64 |
| Citrullus colocynthis | Ag | Polyphenols with aromatic ring and bound amide region | 65 |
| Coleus aromaticus Lour. | Ag | Flavonoids, lignin | 66 |
| Corriandrum sativum | ZnO | Phyto-constituents such as alcohol, aldehyde and amine | 67 |
| Cycas leaf | Ag | Polyphenols, glutathiones, metallothioneins, ascorbates | 68 |
| Cyperus sp. | Ag | Flavones, quinones, organic acids | 69 |
| Datura metel | Ag | Plastohydroquinone, plastrocohydroquinol | 70 |
| Delonix elata | Ag | Phenolic compounds, flavonoids | 42 |
| Desmodium triflorum | Ag | H+ ions produced a long with NAD during glycolysis, water-soluble antioxidative agents like ascorbic acids | 71 |
| Diopyros kaki | Pt | Terpenoids, reducing sugars | 72 |
| Dioscorea bulbifera | Ag | Polyphenols, flavonoids | 73 |
| Dioscorea oppositifolia | Ag | Polyphenols with an aromatic ring and a bound amide region | 74 |
| Eclipta prostrata | TiO2 | Heterocyclic compounds such as flavones | 75 |
| Elettaria cardamomom | Ag | Alcohols, carboxylic acids, ethers, esters, aliphatic amines | 76 |
| Eucalyptus hybrida | Ag | Flavanoid and terpenoid constituents | 77 |
| Euphorbia nivulia | Cu | Peptides, terpenoids | 78 |
| Festuca rubra | Ag | Reducing sugars, antioxidant compounds, ascorbic acid | 79 |
| Gardenia jasminoides Ellis. | Pd | Geniposide, chlorogenic acid, crocins, crocetin | 80 |
| Glycine max | Pd | Proteins, amino acids | 81 |
| Glycyrrhiza glabra | Ag | Flavonoids, terpenoids, thiamine | 82 |
| Hibiscus cannabinus | Ag | Ascorbic acid | 83 |
| Hydrilla sp. | Ag | Flavones, quinones, and organic acids (e.g., oxalic, malic, tartaric, and protocatecheuic acid) | 69 |
| Hydrilla verticilata | Ag | Proteins | 84 |
| Justicia gendarussa | Au | Polyphenol, flavonoid compounds | 85 |
| Lantana camara | Ag | Carbohydrates, glycosides, flavonoids | 86 |
| Leonuri herba | Ag | Polyphenols, hydroxyl groups | 87 |
| Magnolia kobus | Au | Proteins and metabolites (e.g., terpenoids having functional groups of amines, aldehydes, carboxylic acid, and alcohols) | 88 |
| Mentha piperita | Au & Ag | Menthol | 89 |
| Mirabilis jalapa | Au | Polyols | 90 |
| Morinda pubescens | Ag | Hydroxyflavones, catechins | 91 |
| Ocimum sanctum | Ag & Pt | Phenolic and flavanoid compounds, proteins, ascorbic acid, gallic acid, terpenoids, proteins and amino acids | 92, 93 |
| Ocimum tenuiflorum | Ag | Polysaccharides | 94 |
| Parthenium hysterophorus | Ag | Hydroxyflavones, catechins | 95 |
| Pedilanthus tithymaloides | Ag | Proteins, enzymes | 96 |
| Pelargonium graveolens | Ag | Proteins, terpenoids and other bio-organic compounds | 97 |
| Phoma glomerata | Ag | Proteins, amino acids | 38 |
| Pinus eldarica | Ag | Polyphenolic compounds | 24 |
| Pinus resinosa | Pt | Lignin | 98 |
| Piper betle | Ag | Proteins | 99 |
| Piper betle | Pd | Flavonoids, terpenoids, proteins | 100 |
| Piper nigrum | Ag | Proteins | 101 |
| Plumeria rubra | Ag | Proteins | 102 |
| Saraca indica | Au | Polyphenolic compounds | 103 |
| Sesuvium portulacastrum | Ag | Proteins, flavones, terpenoids | 104 |
| Solanum lycopersicums | Au & Ag | Flavonoids, alkaloids, antioxidant vitamins, carotenoids (lycopene), polyphenols | 105 |
| Solanum xanthocarpum | Ag | Phenolics, alkaloids, sugars | 106 |
| Sorghum bicolor Moench | Ag & Fe | Polyphenols | 107 |
| Syzygium aromaticum | Au | Flavonoids | 108 |
| Terminalia arjuna | Cu | Flavonones, terpenoids | 109 |
| Terminalia catappa | Au | Hydrolysable tannins | 110 |
| Terminalia chebula | Ag | Polyphenols present in the form of hydrolysable tannins | 111 |
| Trianthema decandra | Ag | Hydroxyflavones, catechins | 112 |
| Tridax procumbens | CuO | Water-soluble carbohydrates | 113 |
| Zingiber officinale | Au & Ag | Alkanoids, flavonoids | 114 |
2.2. Nano-agriculture: nanobiotechnology applications in crop-science
Nano-pesticides or plant protection products in nano formulation represent an emerging technological development that, in relation to pesticide use, could offer a range of benefits such as increased efficacy, durability, and a reduction in the amounts of required active ingredients.115 Nanofertilizers are able to synchronize the release of nutrients with their plant uptake, thus avoiding the loss of nutrients and reducing the risks of groundwater pollution.116 Biochar, a carbon-rich product, has been shown to suppress plant disease and improve agriculture.117 Moreover, NPs are able to deliver DNA and chemicals into plant cells.118 While carbon nanotubes (CNTs) have been shown to dramatically improve the germination of some comestible plants, deficiencies in behavior consistency and reproducibility have arisen, in part, due to the variability of the CNTs used; they have shown promise as regulators of seed germination and plant growth. Khodakovskaya et al.119 demonstrated that multi-walled CNTs (MWCNTs) enhanced the growth of tobacco cell culture (55–64% increase over control) over a wide range of concentrations (5–500 μg mL−1). Interestingly, they found a correlation between the activation of growth in cells exposed to MWCNTs and the up-regulation of genes involved in cell division/cell wall formation and water transport. The expression of the tobacco aquaporin gene (NtPIP1) and the production of the NtPIP1 protein were significantly increased in cells exposed to MWCNTs in comparison to control cells. The expression of marker genes for cell division (CycB) and cell wall extension (NtLRX1) was also up-regulated in cells exposed to MWCNTs, compared to control cells. Moreover, Giraldo et al.120 showed that single-walled CNTs get passively transported and irreversibly localized within the lipid envelope of extracted plant chloroplasts, thus promoting over three times higher photosynthetic activity than that of controls, and enhancing maximum electron transport rates. The single-walled CNT–chloroplast assemblies also enabled higher rates of leaf electron transport in vivo through a mechanism consistent with augmented photo-absorption. Concentrations of reactive oxygen species (ROS) inside extracted chloroplasts were significantly suppressed by delivering poly(acrylic acid)-nanoceria or single-walled CNT–nanoceria complexes. Furthermore, single-walled CNTs enabled near-infrared fluorescence monitoring of nitric oxide both ex vivo and in vivo, thus illustrating that a plant can be augmented to function as a photonic chemical sensor.53Nano-bionic engineering of plant functions may contribute to the development of biomimetic materials for light-harvesting and biochemical detection with regenerative properties and enhanced efficiency. Chandra et al.121 developed biocompatible amine-functionalized fluorescent carbon dots and isolated them for gram-scale applications. These carbogenic quantum dots can strongly conjugate over the surface of the chloroplast, and due to this strong interaction, the former can easily transfer electrons to the latter by assistance from absorbed light or photons. An exceptionally high electron transfer from carbon dots to the chloroplast can directly affect the whole chain electron transfer pathway in a light-assisted reaction of photosynthesis, where electron carriers play an important role in modulating the system. As a result, carbon dots can promote photosynthesis by modulating the electron transfer process, as they are capable of hastening the conversion of light energy to electrical energy, and finally, to chemical energy, as assimilatory power (ATP and NADPH).
3. Plant-derived nanostructures: types, preparation and applications
Plants have numerous benefits as natural nano-factories. For instance, gliadin NPs have been used as carriers for the oral administration of lipophilic or anticancer drugs. Yi et al.122 developed an infusion-dialysis procedure for isolating spherical tea NPs from green tea with diameters of 100–300 nm and a zeta potential of −26.52 mV at pH 7, and explored the potential of these NPs as multifunctional nanocarriers for cancer therapy in vitro. Daus and Heinze prepared spherical xylan NPs with mean diameters ranging from 162 to 472 nm for drug delivery applications.123 Kung et al.124 used an essential oil from peppermint plants to prepare luminescent NPs; such peppermint oil-derived NPs had a narrow particle size distribution (approximately 1.5 ± 0.5 nm) with prominent blue emission under ultraviolet irradiation. In addition, Johnson-Buck et al.125 reported the synthesis of nanocrystals from indigo dye by re-precipitation using the plant Indigofera tinctoria as a natural source of indigo dye. In one study, Koga et al.126 demonstrated the fabrication of highly transparent conductive networks on cellulose nanopaper. Cellulose nanofibers with a width of approximately 15 nm and a length of more than several μm were extracted from softwood chips (Sitka spruce, Picea sitchensis). The cellulose nanopaper acted as both a filter and a transparent flexible substrate for the silver nanowires and CNTs breaking new ground in the creation of next-generation paper electronics. Gilca et al. presented a physical method to obtain NPs based on lignin by acoustic irradiation.127 Athinarayanan et al.128 synthesized spherical biogenic silica NPs (∼10–30 nm) from acid pretreated rice husks via calcination. Photoluminescence studies indicated that amorphous silica was an appropriate resource for silicon NPs for solar cell or biomedical applications. In another study, Mehta et al.129 reported a one-pot method for the green synthesis of water-dispersible fluorescent carbon dots (∼3 nm size) using Saccharum officinarum juice; they served as excellent fluorescent probes for cellular imaging of bacteria (for example, Escherichia coli) and yeast (Saccharomyces cerevisiae). Yuan et al.130 demonstrated an efficient and low-cost pathway to nitrogen-doped carbon dots by using widely available plant cytoplasm as both the carbon and nitrogen source; it served as a label-free and highly sensitive and selective probe for detecting p-nitroaniline in both, soils and aqueous media. Reddy et al.131 prepared wheat glutenin NPs (∼70–140 nm) for drug delivery applications. Strong acidic or alkaline conditions provided glutenin NPs with low diameters and the particles were more stable under pH 7 rather than a pH of 4.131 Ng et al.132 synthesized equilateral hexagonal EMT-type zeolite NPs from rice husk.The following section aims to provide insight into the use of plants as bio-renewable, sustainable, and diverse sources and as platforms for the production of useful nanostructures and NPs, with applications in various disciplines, including medicine, industry, and agriculture (Table 2).
| Plant-derived nanostructures | Applications | Ref. |
|---|---|---|
| Protein-based NPs | -Controlled drug & gene delivery | 69, 70, 133, 134 |
| -Bioactive compound delivery | ||
| -Tissue engineering | ||
| -Food industry | ||
| -Improvement of oral bioavailability of drugs | ||
| -Drug loaded carriers for medical applications (e.g., gliadin) | ||
| Polysaccharide-based NPs | -Drug delivery systems based on nanocellulose | 135–137 |
| -Drug excipients | ||
| -Blood vessel replacement | ||
| -Soft-tissue-ligament, meniscus & cartilage replacements | ||
| -Nucleus pulposus replacement | ||
| -Tissue repair, regeneration & healing | ||
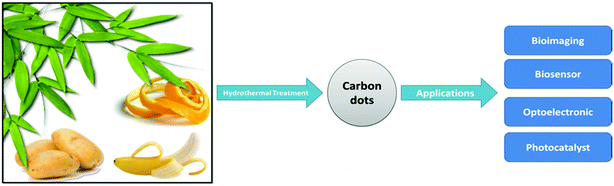
| Carbon-based nanostructures | -Bioimaging | 138–141 |
| -Biosensor | ||
| -Optoelectronic | ||
| -Photocatalyst | ||
| -Electrodes in energy storage devices | ||
| -Organic photovoltaic cells | ||
| -Fluorescent ion detection | ||
| Exosome-like NPs | -Oral delivery | 142, 143 |
| -Modulation of intestinal tissue renewal processes | ||
| -Regulation of gene expression | ||
| Adhesive NPs | -Tissue engineering & biomedical applications | 144, 145 |
| -Platelet aggregation, leading to clotting, & the sealing of wounds | ||
| -Cosmetics | ||
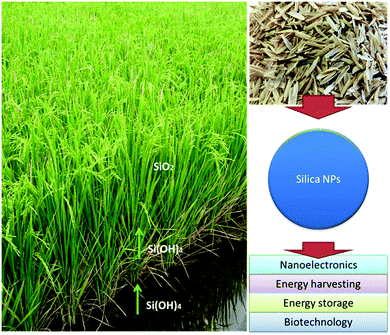
| Silica NPs | -Lithium-ion battery | 146, 147 |
| -Nanoelectronics | ||
| -Photonics | ||
| -Food additive | ||
| -Energy harvesting | ||
| -Energy storage | ||
| -Drug carriers | ||
| -Tissue engineering | ||
| -Anti-caking agent in the food industry | ||
| Lipid-based NPs | -Generation of soft nanomaterials such as nanotubes, nanofibers, gels and surfactants | 148,149 |
| -Biomedical applications |
3.1. Protein-based NPs
Protein bio-macromolecules, comprising a family of L-α-amino acids with different characteristics, perform many functions within living organisms. Proteins consisting four distinct structures can be folded into three-dimensional (3D) structures, the primary structure being the amino acid sequence. The secondary structure consists of regularly repeating local structures stabilized by hydrogen bonds, and the most common examples are the alpha-helix, beta-sheet, and turns. The tertiary structure includes the overall form of a single protein molecule, and the spatial relationship of the secondary structures to one another. The tertiary structure not only is generally stabilized by non-local interactions, most commonly via the formation of a hydrophobic core, but also through salt bridges, hydrogen bonds, disulfide bonds, and post-translational modifications. The quaternary structure, usually called protein subunits in this context, is the structure formed by several protein molecules (polypeptide chains), which acts as a single protein complex. Most of the proteins are enzymes involved in metabolism. Furthermore, there are proteins, such as actin and myosin in muscle, which are responsible for cell structural properties. Additionally, some proteins are important in cell signaling, immune response, cell adhesion, and importantly, there are proteins essential for animals'diet.1333.1.1.1. Zein NPs. Zein is a water-insoluble plant storage prolamine protein from corn (Zea mays L.) that has been used extensively in industrial and food applications such as for coatings of paper cups, in clothing fabrics, adhesives, and binders.152 Upon cast drying, zein treated under acidic conditions formed films with holes (Fig. 4A and B), while zein treated under near neutral and basic conditions formed uniform particles ranging from 100 to 400 nm (Fig. 4C–E). Zein has three quarters of lipophilic and one-quarter of hydrophilic amino acid residues, and consists of three fractions that vary in molecular weight (MWt) and solubility namely α-zein (MWt, 19–24 kDa; 75–80% of total protein), β-zein (17–18 kDa, 10–15%), and γ-zein (27 kDa, 5–10%). A large proportion (>50%) of non-polar amino acids (leucine, proline, alanine, and phenylalanine) in zein make it water insoluble. Zein is deficient in essential amino acids, such as tryptophan and lysine, and hence, is poor in nutritional quality. Commercial zein is available in two grades, yellow and white, and its particulate systems have been prepared using phase separation based on the differential solubility of zein in ethanol and aqueous solution. Zein is approved by the Food and Drug Administration (FDA) as a generally recognized safe excipient for pharmaceutical film coatings. Xu et al.134 developed biodegradable hollow zein to remove reactive dyes from simulated post-dyeing wastewater with a remarkably high efficiency. Hollow zein NPs (ZN) have a higher adsorption for Reactive Blue 19 than solid structures, and the adsorption amount has been shown to increase with a decrease in temperature and pH or an increase in the initial dye concentration. The adsorption capacity of hollow zein has also been shown to be much higher than that of various biodegradable adsorbents developed to remove reactive dyes.
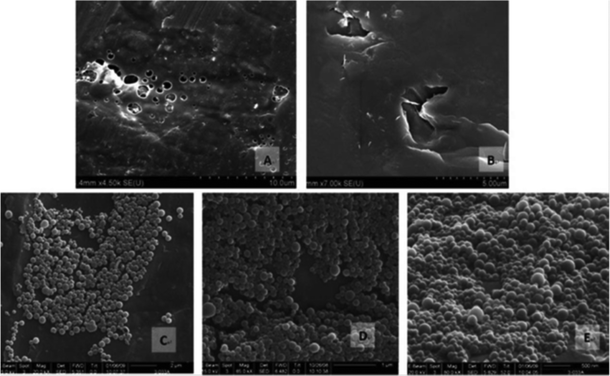 | ||
| Fig. 4 SEM images of pH-treated dried zein samples: (A) Z2.7, (B) Z3.3, (C) Z6.5, (D) Z10.5, and (E) Z12.5 reproduced with permission from ref. 153. | ||
Zein has been used in controlled drug delivery and tissue engineering (Fig. 5), as exemplified by the zein nanofiber-based siRNA delivery system, wherein the amphiphilic property of zein and the size advantage of nanofibers have been brought together. A morphological analysis of the GAPDH-siRNA loaded zein nanofibers revealed the proper encapsulation of the siRNA in the polymeric matrix, the loading efficiency of this delivery system being 58.57 ± 2.4% (w/w). The agarose gel analysis revealed that the zein nanofibers preserved the integrity of siRNA for a longer period even at room temperature. The in vitro release studies not only depicted the sustaining potential of the zein nanofibers but also ensured the release of a sufficient quantity of siRNA required to induce the gene silencing effect. The amphiphilic property of zein supported cell attachment facilitated the transfection of siRNA into the cells; qRT-PCR analysis confirmed the desired gene silencing effect.154 In addition, this biodegradable and biocompatible protein can be used for several industrial applications including agriculture, cosmetics, packaging, and pharmaceuticals. For example, the controlled delivery of hollow NPs from zein to different organs of mice was achieved via cross-linking using starch-derived non-toxic polycarboxylic acid and citric acid. The NPs showed improved stability in an aqueous environment at pH 7.4 without affecting the adsorption of 5-fluro uracil (5-FU), a common anticancer drug, rendering them as potential vehicles for the controllable delivery of anticancer therapeutics.155
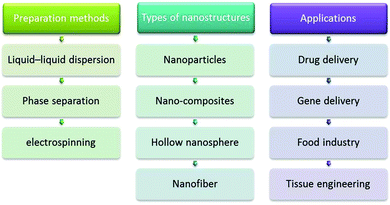 | ||
| Fig. 5 Zein: some important preparations methods, types of nanostructures, and important applications. | ||
In an effort to improve the oral bioavailability of daidzin, an isoflavone glycoside with estrogenic activities, Zou et al.156 designed TPGS 1000 (TPGS) emulsified zein NPs (TZN). ZN and TZN were fabricated as spherical NPs, approximately 200 nm in diameter, with a low polydispersity using an anti-solvent method; the zeta potentials being ∼+25 mV at pH 5.5 and −23 mV at pH 7.4. The addition of TPGS, as an emulsifier, increased the encapsulation efficiency of daidzin in the ZN. The daidzin-loaded TZN exhibited a slower daidzin release when compared with the daidzin-loaded ZN, in both simulated digestive fluids and at pH 7.4 buffer. Confocal laser scanning microscopy suggested that the cellular uptake of coumarin-6-labeled TZN in human intestinal epithelial Caco-2 cells is significantly higher than that of fluorescent ZN. Cellular uptake and transport studies revealed that daidzin in TZN was taken up more efficiently into Caco-2 cells and transported more quickly through a Caco-2 monolayer than through a daidzin solution. A pharmacokinetic study demonstrated that the Cmax of daidzin in mice, after oral administration of daidzin-loaded TZN, was 5.66 ± 0.16 μM, which was a marked (2.64-fold) improvement compared to that of the daidzin solution (2.14 ± 0.04 μM).
Xu et al.134 developed hollow ZN for potential drug delivery applications, with average diameters as small as 65 nm, which were capable of loading a large amount of drugs with the ability to penetrate into the cell cytoplasm; hollow ZN was capable of loading as much as 369 mg g−1 of the drug metformin at an equilibrium concentration of 3 g L−1. Metformin in hollow ZN showed a more sustained and controlled release profile than that in solid ZN; hollow ZN entered fibroblast cells 1 h after incubation. Moreover, Lai et al.157 proposed a new ZN-encapsulated 5-FU that targets the liver via intravenous delivery. The drug loading was optimized and the in vivo targeting efficiency was increased by 31%.
Aswathy et al.158 reported the synthesis of 5-FU-loaded biocompatible fluorescent ZN. ZN, approximately 800 nm in size, was conjugated with quantum dots (ZnS: Mn). NPs were, in turn, encapsulated with the drug 5-FU, and were successfully employed for cellular imaging. Biocompatibility studies showed that NPs at higher concentrations were compatible with cells, and were therefore, expected to be promising agents for targeted drug delivery. In another study, Zhang et al.159 prepared and characterized thymol-loaded ZN stabilized with sodium caseinate and chitosan hydrochloride; encapsulated thymol was more effective in suppressing gram-positive bacterium than un-encapsulated thymol for a longer period. Lee et al.160 introduced a novel drug delivery system composed of zein, and demonstrated that ZN protected therapeutic proteins, catalase and SOD from the harsh conditions found in the gastrointestinal (GI) tract; folate-conjugated catalase or SOD in ZN was able to target activated macrophages, and scavenge the ROS generated by macrophages in vitro. Chen et al.161 produced tangeretin-loaded protein NPs by mixing an organic phase containing zein and tangeretin with an aqueous phase containing β-lactoglobulin. Overall, they demonstrated that protein-based NPs could be used to encapsulate bioactive tangeretin so that it could be readily dispersed in compatible food products.
Luo et al.162 encapsulated a hydrophobic nutrient, α-tocopherol (TOC), into a zein/chitosan complex and its physicochemical and structural analysis showed that electrostatic interactions and hydrogen bonds were the major forces responsible for complex formation. Compared with ZN, the zein/chitosan complex provided a better protection of TOC release under GI conditions, due to the chitosan coatings. Luo et al.163 prepared ZN coated with carboxymethyl chitosan (CMCS) to encapsulate vitamin D3 (VD3) and found that VD3 was first encapsulated into ZN using a low-energy phase separation method, and was simultaneously coated with CMCS. Then, calcium was added to cross-link the CMCS to obtain a thicker and denser coating; NPs with CMCS coatings had a spherical structure, with the particle size ranging from 86 to 200 nm. The encapsulation efficiency was greatly improved to 88% after CMCS coating, compared with 52.2% for those using zein as a single encapsulant. NPs with coatings provided a better controlled release of VD3 in both a phosphate buffered saline (PBS) medium and under simulated GI tract conditions. Photo-stability against UV light was significantly improved as well after encapsulation.
Indole-3-carbinol (I3C) and 3,3′-diindolylmethane (DIM) are two bioactive compounds that are obtained from cruciferous vegetables. However, the stability of these compounds has been a major limitation for their pharmaceutical applications. Luo et al.164 prepared zein and zein/CMCS NPs to encapsulate I3C and DIM with a combined liquid–liquid phase separation and ionic gelation method; the zeta potential decreased from approximately −10 to −20 mV, and the encapsulation efficiency was greatly improved, thus both NP formulations provide a controlled release of I3C and DIM in PBS. Wu et al.165 encapsulated two essential oils (EOs), thymol and carvacrol, in zein NPs using a liquid–liquid dispersion method. Reduction of Escherichia coli from 0.8 to 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU mL−1 was achieved in the presence of NPs encapsulating EOs.
log CFU mL−1 was achieved in the presence of NPs encapsulating EOs.
Regier et al.166 fabricated zein nanospheres encapsulating DNA using a coacervation technique, without the use of hazardous solvents or harsh temperatures, resulting in the preservation of DNA integrity and particles with diameters that ranged from 157.8 ± 3.9 nm to 396.8 ± 16.1 nm, depending on the zein to DNA ratio. The spheres protected encapsulated DNA from DNase I degradation, and exhibited sustained plasmid release for at least 7 days, with minimal burst during the initial release phase thus demonstrating robust biocompatibility, cellular association, and internalization.
Gomez-Estaca et al.167 prepared ZN with a compact spherical structure and a narrow size distribution by electro-hydrodynamic atomization, and showed that ZN could be obtained from zein at concentrations ranging from 2.5% to 15% (w/w). The sizes of these particles, ranging from 175 to 900 nm, increased with increasing polymer concentrations. The morphology of the NPs did not change after incorporating curcumin in proportions ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 to 1
500 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (curcumin
10 (curcumin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zein), and the encapsulation efficiency was approximately 85–90%. Fluorescence microscopy images showed that the ensuing nanostructures were similar in form to the matrix systems, with the curcumin homogeneously distributed in the zein matrix. The curcumin remained in an amorphous state in the NPs, as revealed by X-ray diffractometry, which showed its close contact with the polymer. After extended storage of three months at 23 °C and 43% relative humidity in the dark, neither the size nor the morphology of the NPs had undergone significant changes, and the curcumin content was not affected. Due to the encapsulation, the curcumin was well dispersed when evaluated in an aqueous food matrix of semi-skim milk.
zein), and the encapsulation efficiency was approximately 85–90%. Fluorescence microscopy images showed that the ensuing nanostructures were similar in form to the matrix systems, with the curcumin homogeneously distributed in the zein matrix. The curcumin remained in an amorphous state in the NPs, as revealed by X-ray diffractometry, which showed its close contact with the polymer. After extended storage of three months at 23 °C and 43% relative humidity in the dark, neither the size nor the morphology of the NPs had undergone significant changes, and the curcumin content was not affected. Due to the encapsulation, the curcumin was well dispersed when evaluated in an aqueous food matrix of semi-skim milk.
Jiang et al.168 investigated core-sheath nanofibers prepared using coaxial electro-spinning to provide biphasic drug release profiles. Using ketoprofen (KET) as the model drug, and polyvinylpyrrolidone (PVP) and zein as the sheath polymer and the core matrix, respectively, the coaxial process could be carried out smoothly and continuously without any clogging of the spinneret. In this study, SEM and transmission electron microscopy (TEM) demonstrated that the nanofibers were linear with a homogeneous structure, and had a clear core-sheath structure with an average diameter of 730 ± 190 nm, in which the sheath had a thickness of approximately 90 nm. Differential scanning calorimetric (DSC) and X-ray diffraction (XRD) analyses verified that all the components in the core-sheath nanofibers were present in an amorphous state. Attenuated total reflectance Fourier transform infrared spectra (FTIR) demonstrated that both the sheath and the core matrix had good compatibility with KET due to hydrogen bonding. In vitro dissolution tests showed that the nanofibers provided an immediate release of 42.3% of the contained KET, follow by a sustained release of the remaining drug over 10 h.
Huang et al.169 investigated the preparation of drug-loaded fibers using a modified coaxial electro-spinning process, in which only an unspinnable solvent was used as the sheath fluid. With a zein/ibuprofen (IBU) co-dissolving solution and N,N-dimethylformamide as the core and sheath fluids, respectively, the drug-loaded zein fibers were continuously and smoothly generated without any clogging of the spinneret. Field-emission SEM and TEM observations demonstrated that the fibers had a ribbon-like morphology with a smooth surface. The average fiber diameters were 0.94 ± 0.34 and 0.67 ± 0.21 μm, when the sheath-to-core flow rate ratios were 0.11 and 0.25, respectively. X-ray diffraction and differential scanning calorimetry analyses verified that the IBU was amorphous in all of the fiber composites. FTIR spectra showed that the zein exhibited good compatibility with IBU due to hydrogen bonding. In vitro dissolution tests showed that all the fibers provided sustained drug release profiles via a typical Fickian diffusion mechanism. This modified coaxial electrospinning process could expand the capability of electro-spinning to generate fibers and provide a new method for developing novel drug delivery systems.
Sun et al.170 evaluated the supercritical CO2 anti-solvent technology for preparing ZN loaded with resveratrol wherein it was found that the resveratrol yield was lower when CO2 pressure was increased, while the loading yield was higher with an increased temperature and ratio. The structure of resveratrol-loaded ZN was a matrix with a well-distributed spherical shape and in vitro drug release studies showed that the products exhibited a slower release than resveratrol by itself. Hu et al.171 applied solution-enhanced dispersion by supercritical fluids (SEDS) for the production of lutein/ZN and found that NPs with a high drug loading and a high entrapment efficiency could be prepared using this process. Temperature, pressure, the ratio of lutein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zein, and the solution flow rate influenced the morphology, drug loading, entrapment efficiency, and mean particle size of the lutein/zein NPs. Lower temperature and solution flow rate, coupled with high pressure, favored smaller and more regular-shaped spheres. The initial burst release was hardly observed in NPs processed at 45 °C per 10 MPa. Furthermore, the lutein release profile displayed a near zero-order release, which implied that the NPs played a role in controlled lutein release.
zein, and the solution flow rate influenced the morphology, drug loading, entrapment efficiency, and mean particle size of the lutein/zein NPs. Lower temperature and solution flow rate, coupled with high pressure, favored smaller and more regular-shaped spheres. The initial burst release was hardly observed in NPs processed at 45 °C per 10 MPa. Furthermore, the lutein release profile displayed a near zero-order release, which implied that the NPs played a role in controlled lutein release.
Zou et al.172 fabricated cranberry procyanidins (CBPs)–ZN using a modified liquid–liquid dispersion method. They found that the particle size of the CBP–ZN increased from 392 nm to 447 nm, with increasing CBP-to-zein mass ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to 1
8 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The oligomers with higher degrees of polymerization (DP) showed a higher loading efficiency than the oligomers with lower DPs, suggesting a greater binding affinity of zein proteins. FTIR spectroscopy suggested that the primary interactions between the CBPs and zein were hydrogen bonds and hydrophobic interactions. Cell culture studies, using human promyelocytic leukemia HL-60 cells, showed that the CBPs encapsulated in the NPs had decreased cytotoxicity compared to the CBPs.
2. The oligomers with higher degrees of polymerization (DP) showed a higher loading efficiency than the oligomers with lower DPs, suggesting a greater binding affinity of zein proteins. FTIR spectroscopy suggested that the primary interactions between the CBPs and zein were hydrogen bonds and hydrophobic interactions. Cell culture studies, using human promyelocytic leukemia HL-60 cells, showed that the CBPs encapsulated in the NPs had decreased cytotoxicity compared to the CBPs.
Zhong et al.173 used spray-drying to encapsulate a model antimicrobial of lysozyme in corn zein. The effects of the zein/lysozyme (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and zein/thymol (1
1) and zein/thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 4
0 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratios on the microstructures of the microcapsules, and the in vitro release profiles of the encapsulated lysozyme, were investigated; less lysozyme was released at a higher pH, resulting from the stronger molecular attraction between zein and lysozyme. Nanoscale microcapsule matrix structures were correlated with release characteristics of the encapsulated lysozyme. At intermediate zein/lysozyme (10
1) ratios on the microstructures of the microcapsules, and the in vitro release profiles of the encapsulated lysozyme, were investigated; less lysozyme was released at a higher pH, resulting from the stronger molecular attraction between zein and lysozyme. Nanoscale microcapsule matrix structures were correlated with release characteristics of the encapsulated lysozyme. At intermediate zein/lysozyme (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and zein/thymol (50
1) and zein/thymol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratios, microcapsules had a continuous matrix structure, and revealed a sustained release (11–65%) of lysozyme at pH 6 over 49 days. The nano-scale diameters, biocompatibility, potential for loading a large quantity of drugs, and the ability to penetrate into cells render ZN ideal candidates for transporting various molecules for intracellular drug delivery and tissue engineering for biomedical applications.134
1) ratios, microcapsules had a continuous matrix structure, and revealed a sustained release (11–65%) of lysozyme at pH 6 over 49 days. The nano-scale diameters, biocompatibility, potential for loading a large quantity of drugs, and the ability to penetrate into cells render ZN ideal candidates for transporting various molecules for intracellular drug delivery and tissue engineering for biomedical applications.134
3.1.1.2. Gliadin NPs. Ezpeleta et al.174 have studied the feasibility of preparing small-sized carriers from vegetal macromolecules. For this purpose, they selected gliadin (a vegetal protein fraction from wheat gluten) NPs as drug carriers for all-trans-retinoic acids (RAs). Their systems were prepared by a desolvation method for macromolecules, which produced gliadin NPs of about 500 nm, with a yield close to 90% of the initial protein in environmentally acceptable solvents such as water and ethanol. Moreover, due to the low solubility of this protein in water and its high hydrophobicity, the gliadin NPs did not require any further chemical or physical treatment for hardening. Gliadin NPs were quite stable over 4 days in PBS, but rapidly degraded over 3 h when incubated in a PBS solution containing trypsin. However, chemical cross-linkage of NPs with glutaraldehyde markedly increased their stability. Finally, the in vitro release profiles of RA-loaded gliadin NPs showed a biphasic pattern, where an initial burst effect (in which ∼20% RA was released), followed by zero-order diffusion (release rate 0.065 mg RA h−1) was observed. Arangoa et al.175 reported that gliadin NPs dramatically increased carbazole oral bioavailability up to 49%, and provided sustained release properties pertaining to a decrease of the carbazole plasma elimination rate.
α-Tocopherol or vitamin E is widely used as a strong antioxidant in many medical and cosmetic applications. However, it is rapidly degraded in view of its light, heat, and oxygen sensitivity. Thus, all vitamin E formulations must avoid contact with light, heat, and air. Drug-loaded vitamin E carriers are an attractive option, particularly if they are made of bioacceptable macromolecules, such as vegetal proteins. For instance, gliadins generate NPs by a desolvation method, and may interact with epidermal keratin for therapeutic or cosmetic formulations. Vitamin E-loaded gliadin NPs have been characterized based on their size, zeta potential, vitamin E payload, and entrapment efficiency, and it was shown that the gliadin particle size is ∼900 nm after vitamin E loading, and their charge is close to zero; these gliadin particles are suitable vitamin E drug carriers, with an optimum encapsulation rate of ∼100 μg of vitamin E per mg of gliadin, with an efficiency of more than 77%. The release behavior of vitamin E-loaded NPs has been interpreted as a “burst effect,” followed by a diffusion process through a homogeneous sphere.176
Kajal and Misra177 prepared NPs incorporating tetanus toxoid and a model antigen ovalbumin, and investigated them as delivery vehicles for oral immunization. Gliadin was again used as the carrier because of its biocompatibility, oral bioavailability, and mucoadhesive properties. The size of NPs with ∼50% w/w of antigen remained stable over 3 weeks of testing. Gulfam et al.178 used an electro-spray deposition system to synthesize gliadin and gliadin–gelatin composite NPs for the delivery and controlled release of an anticancer drug (e.g., cyclophosphamide; cyclophosphamide was gradually released from the gliadin NPs for 48 h).
3.1.2.1. Legumin and vicilin NPs. Legumin is a storage protein from Pisum sativum. Mirshahi et al.181 prepared legumin NPs approximately 250 nm in size via a pH-coacervation protocol and chemical cross-linking with glutaraldehyde. However, this organic solvent-free preparation method yielded only approximately 27% of proteins as NPs. In addition, no significant differences in size, percentage yield, or surface charge were observed between the legumin NPs cross-linked with different glutaraldehyde concentrations. The legumin NPs were quite stable in PBS, and they followed a zero-order degradation manner, whereby, a longer half-life (t50) was obtained with increasing glutaraldehyde concentrations. The amount of methylene blue (MB), used as a model of a hydrophilic drug, loaded was approximately 6.2% of the initial dye. Its release from the NPs consisted of a rapid initial phase, followed by a slower second period, in which the rates in the second phase were inversely related to the degree of cross-linking.
3.1.2.2. Glycinin and β-conglycinin NPs. Soybeans (Glycine max L.) are currently one of the most abundant sources of plant proteins. The enriched form of soy protein, known as soy protein isolate (SPI), has been reported to unveil high nutritional values and desirable functionalities; its wide application as a food ingredient has been well documented. SPI also possesses a balanced composition of non-polar, polar, and charged amino acids; thus, drugs can be incorporated with their various functional groups. The major components of SPI are glycinin (MWt = 360
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, ∼60%) and β-conglycinin (MWt = 180
000, ∼60%) and β-conglycinin (MWt = 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, ∼40%). Teng et al.182 successfully encapsulated curcumin, as a model drug, into NPs, and the average size of the curcumin-loaded NPs ranged from 220.1 to 286.7 nm, and their zeta potential was approximately −36 mV. The highest encapsulation efficiency and loading efficiency achieved in their study were 97.2% and 2.7%, respectively.
000, ∼40%). Teng et al.182 successfully encapsulated curcumin, as a model drug, into NPs, and the average size of the curcumin-loaded NPs ranged from 220.1 to 286.7 nm, and their zeta potential was approximately −36 mV. The highest encapsulation efficiency and loading efficiency achieved in their study were 97.2% and 2.7%, respectively.
3.2. Plant polysaccharide-based nanostructures
At first glance, the relationship between nanotechnology and lignocellulosic biomass may seem unrelated. However, it is important to recognize that, at a fundamental level, lignocellulosic biomass is comprised of nanoscale building blocks that provide valuable properties to wood and other types of renewable cellulosic and lignocellulosic biomaterials.183The great potential of renewable biomaterials has been previously overlooked. Trees and plants use air, sunlight, and water to conduct processes in their “photochemical factories,” and generously produce naturally occurring nanocomposites of cellulosic microfibrils embedded in a lignin matrix.186 As a result of recent insight into this natural gift, the great potential of lignocelluloses as nanomaterials has come into limelight. Their unique nanocellulosic structure can be isolated by a top-down approach and custom-made into well-defined architectures that are sustainable, renewable, recyclable, and environmentally friendly.136
3.2.1.1. Cellulose. Cellulose is a fibrous, semi-crystalline polymer of high molar mass and is the most abundant biopolymer on earth187 which is a renewable, carbon-neutral, biodegradable, sustainable, and inexpensive raw material (Fig. 6). It is the main constituent of plant structures, where its extended chain conformation and microfibrillar morphology contribute to its significant load-carrying capability.137 Cellulose is found in plants in the form of microfibrils, which comprise the structurally strong framework of cell walls; microfibrils are bundles of cellulose (C6H10O5) molecules that are elongated and stabilized laterally by hydrogen bonds. A single microfibril contains multiple elementary fibrils consisting of many cellulose chains. The typical diameter of elementary fibrils is between 2 and 20 nm, and is reportedly affected by many factors, such as source, variety, soil, climate, harvest, and maturity, etc.188–192 The Young's modulus and tensile strength of cellulose nanofibrils have been reported to be approximately 145 GPa and 7.5 GPa, respectively,189,193,194 and the high modulus of the cellulose microfibril has been attributed to the molecular alignment and dense packing in the unit cell.137
Nanocellulose possesses remarkable physical properties, special surface chemistry and excellent biological properties. Abundant availability, biodegradability, biocompatibility and low toxicity are the main advantages of nanocellulose. Nanocellulose-based nanocomposites have shown potential for applications in drinking water filtration, in the catalytic degradation of organic pollutants, as absorbents to remove trickled oil from water, for censoring organic contaminants and waterborne pathogens, and in exceptional energy conversion devices. Nanocellulose exists in a number of forms such as those that are commonly described as microfibrillated cellulose or nanofibrillated cellulose (homogenized cellulose pulps), nanocrystalline cellulose or cellulose nanocrystals (acid hydrolyzed cellulose whiskers), and bacterial cellulose (Fig. 7). Microfibrillated cellulose is often produced by a high-shear mechanical treatment of micron-sized cellulose pulp that has been pre-treated with bleaching agents to remove lignin and hemicellulose; typical mechanical treatments being refining and high pressure homogenization. In addition to mechanical homogenization, ultrasonic techniques have also been found to be useful for microfibrillated cellulose preparation. In one study, cellulose pulp was pretreated with endoglucanases before homogenization. The resulting nanocelluloses contained both 10–20 nm MFCs and 5 nm microfibrils. Because of the mild enzymatic hydrolysis prior to mechanical treatment, the required energy input was greatly reduced. Nanocrystalline celluloses are highly crystalline nanocelluloses with lower aspect ratios than microfibrillated celluloses and can be prepared by mineral acid hydrolysis of plant cellulose pulp, filter paper, and bacterial cellulose.195 Nanocellulose and its properties have been reviewed comprehensively by Dufresne.196,197
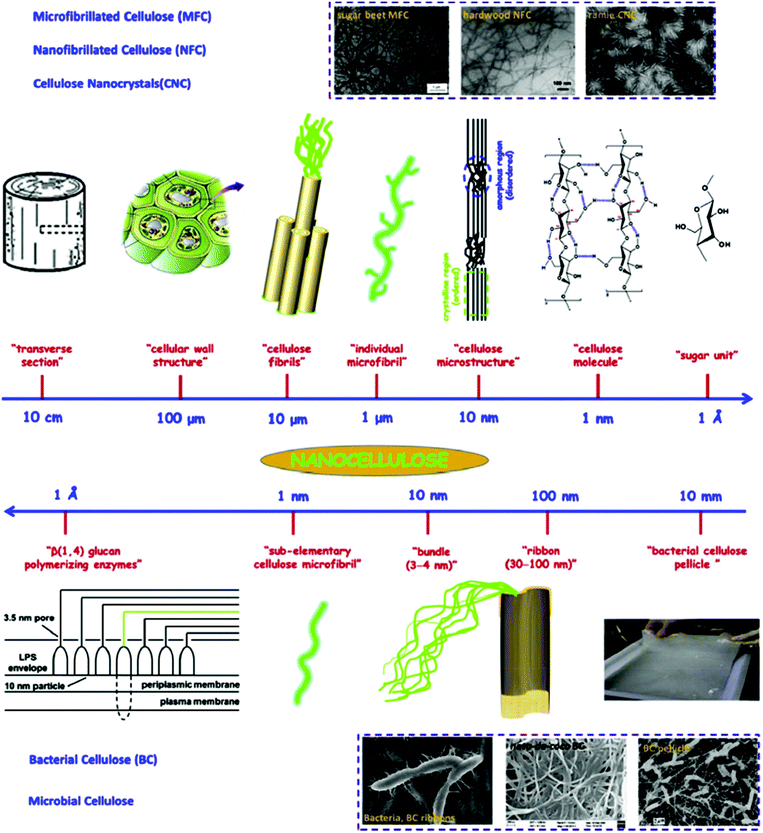 | ||
| Fig. 7 Hierarchical structure of cellulose; top image (from large unit to small unit): cellulose nanocrystals (CNC), micro/nanofibrillated cellulose (MFC and NFC); bottom image (from tiny unit to small unit): bacterial cellulose (BC). Transmission electron micrographs of sugar beet MFC, hardwood MFC, ramie CNC, and scanning electron micrographs of BC ribbons, nata-de-coco BC, and BC pellicle. Reproduced with permission from ref. 135. | ||
Hierarchical structure
Natural fibers, such as wood, are cellular hierarchical bio-composites (Fig. 8). At the nanoscale level, natural fibers are a cellulosic-fibrillar composite. Nanofibrils in their simplest form are elementary cellulosic fibrils, containing both crystalline and amorphous segments, and can be hundreds to a thousand nanometers long. This hierarchical structure, based on elementary nanofibrillar components, is responsible for the unique strength and high performance properties of different species of wood and natural fibers.186
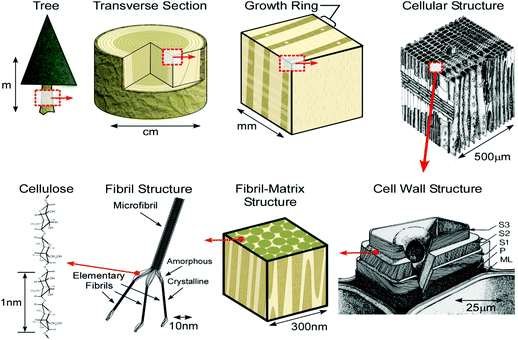 | ||
| Fig. 8 Schematic of the tree hierarchical structure and natural fibers. Reprinted with permission from ref. 198. | ||
Nanotechnology provides the tool for isolating nanocelluloses from natural sources and most of the methodologies for the isolation of cellulose nanofiber require the use of a combination of chemical, mechanical, and other processes. The resulting cellulose nanofibers could have different morphologies, such as an entangled network (nanofibers) or rod-like NPs (whiskers). Increasing the crystallinity or reducing the amorphous region of fibers, by removing the hemicelluloses and lignin, has been shown to effectively increase the cellulose content, and as a result, the fibers had a much higher strength. However, notably, lignin and hemicellulose removal could have detrimental effects in this regard, depending on how it is performed, as the removal could lead to partial cellulose degradation.198 The elastic moduli of solid wood, single pulp fiber, microfibrils, and crystallites are 10 GPa, 40 GPa, 70 GPa, and 250 GPa, respectively.199 Thus, breaking down the cellulosic fibers to the micro- or nanoscale significantly improved the strength of the resulting fibers.200
3.2.1.2. Potential sources. Wood-based resources
Wood-based resources can be used as a source of cellulose and for nanocellulose isolation. Cellulosic nanofibers have been successfully isolated from wood using a combination of chemo-mechanical treatments.201–203
Non-wood crops
There are several advantages in using agricultural residues and natural fibers, over wood-based resources, because wood is a valuable commodity for use in the construction and furniture industries, as well as in the pulp and paper sector. These demands have increased pressure on wood supplies for nanocellulose production, and consequently, interest in agricultural residues and natural fibers e.g., flax, hemp, jute, kenaf, sisal, and others, has grown. The other advantages of using plant fibers include the pace of their renewability in comparison to wood, the reduced lignin content that makes them easier to process, and defibrillation with a decreased energy demand is another salient feature.204
Researchers have used various agricultural residues as sources for nanocellulose production, e.g., rutabaga,205 wheat straw,206,207 bamboo,208 potato tubers,209 hemp,210,211 soybean stock,212 sugar beet pulp,213–216 bagasse,217,218 sugarcane bagasse,219 cottonseed linter,220 flax,221 ramie,222 pea hull,223,224 kenaf,136,200,225–229 wheat straw and soy hulls,230 oil palm empty fruit bunch,230–232 swede root,233 sisal,234 jute,235,236 banana rachis,237 rice husk,238,239 coconut husk,240,241 and Mengkuang leaves.242
Bacterial source
Certain bacteria, belonging to the genera Acetobacter, Agrobacterium, Alcaligenes, Pseudomonas, Rhizobium, or Sarcina,243 biosynthesize cellulose or secrete cellulosic fibers extracellularly. There are several morphological differences between bacterial cellulose and extracted nanocellulose from wood or plants, e.g., it produces ribbon-shaped fibrils, less than 100 nm wide, which are composed of much finer 2–4 nm nanofibrils.244,245 Celluloses produced from bacteria offer certain exceptional properties, such as a fine and pure fibrous network structure, as well as greater mechanical strength; bacterial cellulose has been used as a source of nanocellulose.246–254
3.2.1.3. Isolation techniques for nanocellulose. Isolation of cellulosic nanofibers or crystalline whiskers usually requires a multi-stage process involving vigorous chemical and/or mechanical procedures. Lignin impedes the separation of wood into its component fibers, so it is reasonable to consider delignification methods as promising initial steps for the preparation of nanocellulose.255 These chemical processes aim to produce purified cellulose, such as bleached cellulose pulp, which can then be further processed. Wood pulp, prepared by chemical methods, can be defibrillated by various mechanical methods or further, via acidic hydrolysis. These techniques are discussed in detail below.
Mechanical approaches
Homogenizing: This process occurs in a special homogenizing valve, which is the heart of the equipment, wherein the fluid passes through a gap in the homogenizing valve. This creates conditions of high turbulence and shear, combined with compression, acceleration, pressure drop, and impact, which causes the disintegration of particles.256 For this purpose, a diluted suspension of refined cellulosic fibers is fed into the machine and to achieve nanoscale cellulosic fibers, this homogenization process must be repeated several times which varies significantly throughout the literature. This inconsistency could be due to the degree of pre-treatment and refining processes performed prior to the homogenization process, the raw material used, the fiber length, and the applied pressure. For example, Jonoobi et al.226 reported the isolation of nanofibers from kenaf bast by using a homogenization process with 40 cycles at 500 bar, while Iwamoto et al.257 reported that 14 times is the optimum number of repetitions, and described that further repetitions up to 30 cycles did not improve fibrillation. Malainine et al.258 also reported that 15 cycles at 500 bar were enough for defibrillating the fibers to nanoscale dimensions. Overall, most researchers have subjected the fibers through a homogenizer for approximately 10–20 cycles,259–262 with the common disadvantages of this mechanical treatment being the high energy demand and other issues associated with system clogging due to the processing of long fibers.
Cryocrushing: Cryocrushing is a technique presented by Chakraborty et al.,201 where the water in pulped fibers was frozen using liquid nitrogen, and high shear forces were then applied to force disintegration of the fibrils from the cell wall. Application of high impact forces to the frozen fibers by ice crystals exerts pressure on the cell walls, causing them to rupture, which leads to the liberation of the microfibrils.212 The nanofibers from wheat straw and soy hulls have been extracted by a mechanical approach involving cryocrushing;230 this technique enables the isolation of nanoscale cellulosic fibers from chemically treated hemp, rutabaga, and flax fibers, and nanofibers with diameters ranging from 5–80 nm were attained.263 Wang and Sain212,264 combined this technique with high-pressure homogenization to extract nanofibers from soybean stock of diameters ranging from 50 to 100 nm. The main disadvantage is the non-scalability of this method and since this method does not produce very fine fibrils, it is limited to cellulose fibrils from primary cell walls.265
Grinding or microfluidization: In this method, a suspension of cellulosic pulped fibers is passed through a static grindstone and a rotating grindstone, revolving at a high speed, e.g., 1500 rpm.265,266 The fibers’ cell walls, consisting of nanoscale building blocks and hydrogen bonds, are broken down by shearing forces generated by the grinding stones, which leads to the liberation of nanoscale cellulosic fibers. Karimi et al.136 obtained nanofibers from kenaf bast with diameters ranging from 2.2 to 34 nm by using this technique. When homogenized cellulosic pulp was subjected to a grinder treatment, the fibril bundles were further fibrillated and 10 repetitions of the grinder treatment resulted in uniform nanofibers that were 50–100 nm wide.257,267
In contrast to homogenization, processing of cellulosic fibers with a microfluidizer reduces the likelihood of clogging because it has no in-line moving parts,265 and if clogging occurs, it can be resolved by using reverse flow through the chamber. The main disadvantage of microfluidization has been the maintenance of the disk and disk replacement since wood pulp fibers can rapidly wear down the grooves and grit. However, a primary advantage is that the mechanical fiber shortening pre-treatment, utilized with other processing techniques, may not be required.
Pre-treatments
Mechanical approaches for nanocellulose isolation have two drawbacks, namely high-energy consumption and long fiber lengths that cause clogging of the equipment. Mechanical, chemical, and enzymatic pre-treatment processes can significantly decrease the energy consumption and reduce fiber size, thereby reducing the frequency of equipment clogging. Mechanical pre-treatment: Mechanical reduction of fiber size can be performed using PFI mills, valley beaters, and disk refiners, and by manual cutting. Usually these pre-treatment methods are used prior to the production of nanofibers, i.e., prior to the homogenization or microfluidization process.256,257,268
Alkaline chemical pre-treatment: Alkaline pre-treatment is the most commonly used technique, as it promotes fiber swelling that makes the defibrillation process easier and less energy consuming. Several researchers have used alkaline pre-treatment prior to the main defibrillation process.211,212,214,226,228,242,264,269,270 The purpose of the alkaline treatment is to dissolve and eliminate lignin and hemicellulose from the matrix surrounding the cellulose microfibrils, rendering them vulnerable for the isolation of individual microfibers. However, the process needs to be carefully controlled to avoid undesirable cellulose degradation.212,263 Abe et al.266 produced nanofibers from wood powder, with only one pass through a grinder, following an extensive chemical pre-treatment.
Oxidative chemical pre-treatment: Oxidation pre-treatment, also known as a TEMPO-mediated oxidation process, deploys radicals emanating from 2,2,6,6-tetramethylpi-peridine-1-oxyl (TEMPO) on the substrate prior to the main defibrillation treatment; this technique has proven to be promising for the surface modification of native celluloses.216,271–276 Uniform nanofibers with diameters of 1–5 nm have been isolated via oxidation using nitroxyl radicals from TEMPO277 and the nanofibers possess the same crystallinity as the starting materials;276 when used in conjunction with mechanical treatments, over 90% yield was achieved.278 Cellulose nanocrystals and nanofibrils could be generated from pure rice straw cellulose via sulfuric acid hydrolysis, mechanical blending, and TEMPO-mediated oxidation.279 Interestingly, the TEMPO-mediated oxidation procedure produced the most uniform and finest (approximately 1.7 nm) nanofibers, but the nanofibers were found to be the least crystalline.
Enzymatic pre-treatment: Cellulose enzymes have been suggested to favor the attack on the amorphous region of the cellulosic substrates, making it easier to separate the material into microfibrillated cellulose. A reduction in energy demand has been observed when enzymatic pre-treatment was applied prior to the mechanical treatments.268,280,281 The isolation of cellulose microfibrils by treating bleached kraft pulp, using enzymatic approaches, led to a significant reduction in fiber diameters;282 nanofibers from wood, subjected to enzymatic pre-treatment, resulted in a more favorable structure than wood subjected to strong acidic hydrolysis.283
Acid hydrolysis: Cellulose fibers and microfibrils do not display a regular surface, i.e., apart from the crystalline domains, cellulose also occurs in a non-crystalline or amorphous state; amorphous regions are randomly oriented in a spaghetti-like arrangement, leading to a lower density in comparison to nano-crystalline regions.284,285 The amorphous regions are susceptible to acid attack and, under controlled conditions, they may be removed, leaving crystalline regions intact.286 A strong acid, usually sulphuric acid, is used to hydrolyse cellulose which is immediately followed by quenching of the reaction with deionised water. In order to attain higher concentrations of the cellulose and to remove the extra aqueous acidic solution, the suspension is centrifuged. Cellulosic nanocrystals from kenaf,228,229 bagasse,218 cottonseed linter,220 cotton,287 flax,221 hemp,210 ramie,222 pea hull,223,224 and tunicate288–290 have been isolated by using this acidic hydrolysis.
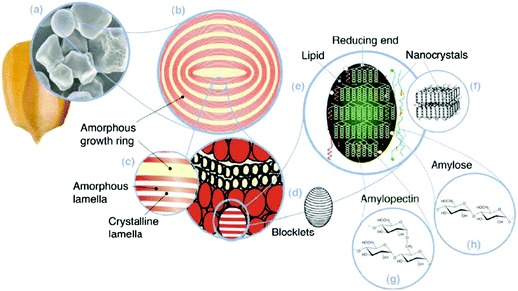 | ||
| Fig. 9 Starch multiscale structure: (a) starch granules from normal maize (30 μm), (b) amorphous and semicrystalline growth rings (120–500 nm), (c) amorphous and crystalline lamellae (9 nm), magnified details of the semicrystalline growth ring, (d) blocklets (20–50 nm) constituting a unit of the growth rings, (e) amylopectin double helixes forming the crystalline lamellae of the blocklets, (f) nanocrystals: other representation of the crystalline lamellae called starch nanocrystals when separated by acid hydrolysis, (g) amylopectin's molecular structure, and (h) amylose's molecular structure (0.1–1 nm). Reproduced with permission from ref. 298. | ||
Starch granules have a 3D architecture with varying crystallinity from 15% to 45%,293 and consist of D-glucose units with two major biomacromolecules, including amylose and amylopectin (Fig. 10). Amylose is a sparsely branched carbohydrate while amylopectin is a highly multiple-branched polymer, with a high molecular weight responsible for the materials’ crystallinity.294 Depending on the source, the amylose content of starch can vary from less than 1% to 83%,295 and its content can significantly affect the thermal, rheological, and processability properties of starch-based materials.296,297
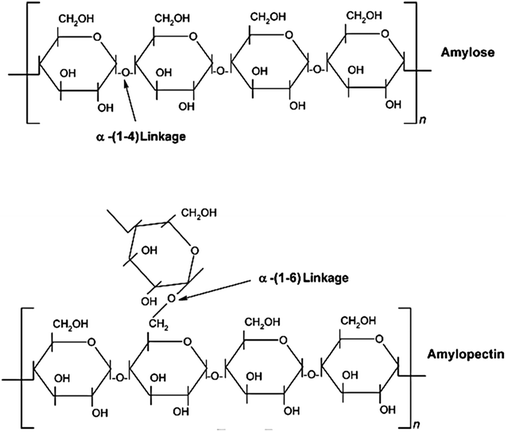 | ||
| Fig. 10 Chemical structure of starch with amylose and amylopectin units. Reproduced with permission from ref. 292. | ||
Starch nanocrystals
Starch crystallites, starch nanocrystals, microcrystalline starch, and hydrolyzed starch all stand for the crystalline part of starch obtained via hydrolysis, but to different extents.298 Initial interest in starch nanocrystals was triggered in 1996 by Dufresne et al.,299 when they described the isolation of microcrystalline starch with diameters in the range of a few tens of nanometers via acidic hydrolysis. Later in 2003, Putaux et al.300 extracted nanocrystals with diameters of 15–30 nm and lengths between 20–40 nm from waxy maize starch by acid hydrolysis. Recently, the isolation of starch nanocrystals from potatoes,301–303 peas,304,305 and waxy maize306–308 has been reported, while the diameter of nanocrystals has been shown to vary within the range of 40–150 nm; this variation could be due to the starch source and its botanical origin.309
3.2.2.1. Preparation techniques for starch nanocrystal. There are two prevailing processes for starch nanocrystal isolation, regardless of starch type and the origin and both techniques are based on acidic hydrolysis; the first method introduced by Dufresne et al.299 involved hydrolysis by hydrogen chloride while the second method was described by Angellier et al.310 and used hydrolysis by sulfuric acid. A schematic of different pathways for producing crystalline and amorphous starch NPs is depicted in Fig. 11.
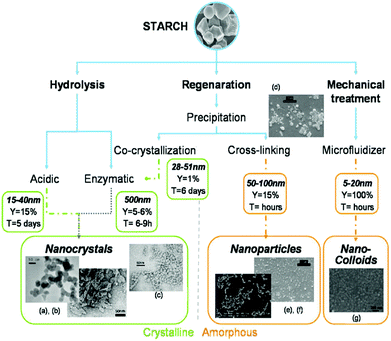 | ||
| Fig. 11 Different ways of producing crystalline and amorphous starch NPs: hydrolysis leads to nanocrystals, whereas regeneration and mechanical treatment lead to both amorphous and crystalline particles in the final batch. (a, b) TEM micrograph of starch nanocrystals. Enzymatic hydrolysis is used to selectively keep crystalline particles: (c) TEM micrograph of starch nanocrystals after cocrytallisation and enzymatic hydrolysis. The production of starch NPs by precipitation of gelatinized starch in non-solvent followed by a cross-linking reaction: (d) precipitated starch NPs before cross-linking, (e) starch NPs and (f) citric acid cross-linked starch NPs. By analogy with microfibrillated cellulose (MFC): (g) TEM micrograph of starch nanocolloid. Reproduced with permission from ref. 298. | ||
Compared to cellulose nanocrystals, the reinforcing capacity of starch nanocrystals is rather limited. Consequently, it should be used in a higher amount to reach similar reinforcing effects. However, other interesting properties can be obtained from their platelet-like morphology like barrier properties. Yet another limitation of starch nanocrystals is the length of the hydrolysis process which is much longer than for cellulose and needs to be performed at lower temperatures owing to the higher sensitivity of starch to the acid assault.298
The low thermal stability is yet another restricting factor that limits the use of lignocellulosic fibers in material composites. To avoid fiber degradation during processing, the temperature is limited to 200 °C, which further restricts the choice of the polymer matrix materials. Moreover, studies on the use of laboratory-scale processing methods such as solution casting have illustrated cellulose nanocomposites in terms of their morphological, physical, and mechanical properties. However, the development of new industrially viable processing techniques is crucial to promote the commercial applications of these materials.
In contrast to the cellulosic nanostructures, significantly fewer studies evaluating starch nanocrystals are available. One of the main interests for the use of starch, in addition to low cost, is that the raw material is relatively pure and does not need an intensive purification procedure such as lignocellulosic materials.298 Consequently, in view of the lower crystallinity of starch nanocrystals than that of nanocelluloses, their reinforcing potential is lower and they need to be used in higher amounts to attain similar reinforcement effects. However, their unique platelet-like morphology introduces some valuable features, such as barrier properties. The disadvantage of starch nanocrystals is that their isolation requires a much longer hydrolysis time in comparison to cellulose.
Moores et al.312 demonstrated an unprecedentedly high enantiomeric excess for Pd NPs deposited onto cellulose nanocrystals which were used as catalysts for the hydrogenation of prochiral ketones in water at room temperature; cellulose nanocrystals served as the support and the chiral source, delivering an enantiomeric excess of 65% with quantitative conversions via binding of Pd NPs onto cellulose nanocrystals thus providing an insight into the chiral induction mechanism.
3.3. Carbon-based nanostructures from plants
Carbon is an essential and abundant universal chemical element with several allotropic forms with unique properties. The best-known forms are amorphous carbon, graphite, diamond, and fullerenes. Approximately 1900 gigatons of elements are present in the biosphere and form the basis of all known life on Earth, and without these elements, life could not exist. Carbon can form very vital bonds with hydrogen and oxygen to build carbohydrates and various other groups of important biological compounds. When associated with nitrogen, sulfur, and phosphorus, carbon forms amazing and infinite biomolecules, including lipids, alkaloids, antibiotics, amino acids, DNA, RNA, ATP, lignans, chitins, alcohols, fats, aromatic esters, carotenoids, terpenes, and rubber products. Plants draw carbon dioxide from their environment and use it to build biomass, as in carbon respiration or the Calvin cycle, a process of carbon fixation. Plants, therefore, are the key carbon source for producing carbon nanostructures.138Several researchers have prepared carbon-based nanostructures from plants.313–316 Wang et al.317 used microwave (MW)-assisted pyrolysis to fabricate porous carbon nanostructures from biomass precursors (e.g., wood, cotton, and filter paper) filled with a conducting polymer and iron (Fe) catalytic species. The morphology and porosity of the biomass precursors were retained, but their infrastructure became highly graphitic after the MW irradiation treatment. The conducting polymer served as a MW absorbent, while the Fe species catalyzed the polymerization of the pyrrole in the first step, as well as expedited pyrolysis of the biomass precursor during MW irradiation. The ensuing graphitic carbon materials possess relatively high surface areas with openly accessible pores. The building blocks of the porous materials have changed from natural polymer tissues to various graphitic carbon nanostructures, including nanofoams, nanoflakes, nanoribbons, and sponge-like nanosheets. Saxena and Sarkar318 isolated carbon NPs (CNPs) from bread. A simple oxidative treatment rendered them water-soluble and fluorescent, and the CNPs exhibited fluorescence over a broad range of excitation wavelengths. They have been successfully used for fluorescence imaging of human erythrocytes under 488 and 561 nm band pass filters. Upon direct interaction with human erythrocytes, these CNPs showed ∼6% hemolysis in 5 h. In the presence of blood plasma, the quantum yield of these water-soluble CNPs was enhanced from 2% to 4.5%, giving rise to a new phenomenon of auto passivation of CNPs by bio-fluids.
Muramatsu et al.139 demonstrated a new synthesis method for transforming rice husks into bulk amounts of graphene via calcination and chemical activation. The bulk sample comprises crystalline nano-sized graphene and corrugated individual graphene sheets; the material generally contains one or more layers, and corrugated graphene domains were typically observed in monolayers containing topological defects within the hexagonal lattice and edges. Both types of graphenes exhibit atomically smooth surfaces and edges.
The oral ingestion of a fluorescent probe is a novel approach for imaging living species. Ghosh et al.319 have introduced water-soluble carbon nano-onions (wsCNOs) as non-toxic, fluorescent reagents enabling the live imaging of Drosophila melanogaster (fruit flies). Water-soluble CNOs, synthesized from wood waste, colorfully image all the developmental phases of Drosophila melanogaster, from the egg to adulthood. Oral ingestion of up to 4 ppm of soluble CNOs allowed optical fluorescence microscopy imaging of all stages of the fruit fly life cycle without exhibiting any toxic effects; fluorescent Drosophila melanogaster excretes this fluorescing material upon the removal of the CNOs from its food. Sonkar et al.320 reported the in vivo effects of wsCNOs introduced in the common food web of two model organisms: unicellular E. coli and multicellular Caenorhabditis elegans. At first, CNOs were fed to E. coli; subsequently, the E. coli were fed to C. elegans. The wsCNOs were found to serve as highly fluorescent bio-imaging agents and the results did not indicate any toxic effects caused by the wsCNOs on the growth of these organisms.
Sonkar et al.321 reported that wsCNOs isolated from wood wool, a wood-based pyrolysis waste product of wood, enhanced the overall growth rate of gram (Cicer arietinum) plants. Treatment of plants with up to 30 μg mL−1 of wsCNOs for an initial 10-day period under laboratory conditions led to an increase in the overall growth of the plant biomass. Analysis of the carbon, hydrogen, and nitrogen content for the shoot and fruit sections of the plants, treated with and without the wsCNOs, showed only a minor difference in the compositions. However, a slight increase in the percentage of carbon and hydrogen in the shoots reflects the synthesis of more organic biomass in the treated plants.
Chen et al.140 reported the synthesis of carbon nanofibers on activated carbons produced from agricultural waste using chemical vapor deposition. Importantly, Fe already present in the ash content of the activated carbon was employed as a natural catalyst for nanofiber formation, and the need for a wet chemical catalyst preparation step was thus avoided. Notably, Zhu et al.141 reported the synthesis of MWNTs from bamboo charcoals by chemical vapor deposition in the presence of ethanol vapor; Mg2SiO4 and, particularly, calcium silicate were responsible for the nucleation and growth of CNTs at 1200 °C–1400 °C. TEM and EDS examinations showed that the tips of nanotubes synthesized at 1200 °C–1400 °C consisted mainly of calcium silicate, thereby acting as effective catalysts for the nucleation of nanotubes. The CNT formation was found to occur following the vapor–liquid–solid (VLS) mechanism, which includes the initial decomposition of ethanol vapor into carbon, dissolution of carbon inside molten silicate, and the final nucleation of CNTs. Furthermore, the upload of CNTs in bamboo charcoals markedly increased the specific surface area from 98 to 655 m2 g−1 and the adsorption capacity from 0.05 to 0.35 cm3 g−1. The CNT growth by silicate may permit applications in cement, in which the production temperature is normally ∼1450 °C, and the silicate inside the cement could be a catalyst for the growth of CNTs.
Goodell et al.324 reported the synthesis of CNTs from wood fiber using a low-temperature process, which included continuous oxidation at 240 °C and cyclic oxidation at 400 °C; the inside diameter of the ensuing CNTs was ∼4–5 nm while the outside diameter ranged from 10 to 20 nm. In contrast, no CNTs were produced when pure lignin and cellulose were evaluated, indicating that the molecular and spatial arrangements of the cell wall played an important role in CNT formation. This study suggested that the chemical components in the secondary plant cell wall, and their differential ablation properties, were critical for the formation of CNTs at these comparatively low temperatures.
The sustainability of any civilization depends on its energy resources, and the technological capital for their conversion and conservation. Paul et al.325 reported the synthesis of CNTs from plant-based precursors and their application in organic photovoltaic cells and bio-diesel storage. Ye et al.326 reported the synthesis of a series of carbon nanostructures via a biotemplating method by catalytic decomposition of bamboo impregnated with ferric nitrate. The natural nanoporous bamboo was used as both a green carbon source and a template for the in situ growth of carbon nanostructures; SEM, field emission TEM, and EDS were used to characterize the product. Four distinct structural types of carbon nanostructures were identified, namely nanofibers, hollow carbon nanospheres, herringbone, and bamboo-shaped nanotubes. The effect of reaction temperature (600 °C–900 °C) on the growth behavior of carbon nanostructures was investigated, and the corresponding growth mechanism was proposed; production of nano-fibers was favored at low temperatures, while higher temperatures led to bamboo-shaped nanostructures.
Xie et al.327 produced CNTs from plant materials using a cyclic oxidation process, after the raw materials were pretreated by oxidative carbonization in air at ∼240 °C. The cyclic oxidation process was found to be more effectual than a continuous heating process in ablating the residual carbon from cellulose. The results also indicated that no CNTs were produced unless a pre-oxidation process was used, or when the cyclic oxidation temperature increased above 600 °C. In another study, Zhao et al.328 used black Jew's-ear fungus and black sesame seeds as catalyst precursors to prepare CNTs by chemical vapor deposition. Each catalyst particle arose from the metal content of a single cell of the precursor, hence the distribution of catalyst particles was uniform; their size and composition were almost identical. CNT arrays grew when black sesame seeds were used as catalyst precursors. CNTs with diameters of 80 nm and lengths greater than 100 μm grew when black Jew's-ear fungus was used.
Dubrovina et al.329 reported the one-pot synthesis of CNTs by pyrolysis of the cellulose acetate (CA) cross-linked with polyisocyanate in a fumed silica template; NiCl2 was chosen as the pre-catalyst for CNT growth. The diameter of the generated CNTs was 24–38 nm, and their wall thickness was 9–11 nm. The primary role of CA pyrolysis in the formation of CNTs may be to combine closed macropores in the template formed by CO2 evolved during the cross-linking reaction and mesopores formed by silica particles; macropores acted as microreactors, while the mesopores templated the catalytic NPs. The importance of this method for CNT synthesis is based on the utilization of a readily available renewable resource of CA. The technologically simple and energetically efficient method can be performed in a conventional tube furnace and does not require the preliminary synthesis of a catalyst.
Green luminescent water-soluble oxygenous C-dots with an average size of 3 nm have been synthesized by simply heating banana (Musa acuminata) juice at 150 °C for 4 h, without using any surface passivating and oxidizing agents, or inorganic salt.332 The ensuing C-dots offered an excitation wavelength and pH-dependent luminescent behavior in the visible range; the quantum yield was 8.95 on excitation at a wavelength of 360 nm, using quinine sulfate as the reference. In another study, dual-emission C-dots have been prepared via pyrolysis and the MW treatment of naked oats, providing a novel and sustainable pathway for the production of dual luminance C-dots without the requirement of a tedious synthetic methodology or the use of toxic/expensive solvents and starting materials;333 their intriguing dual fluorescence behavior comes to the limelight at an excitation wavelength between 250 and 310 nm. The well-resolved dual emission bands manifest excitation and temperature dependence. The produced C-dots were applied as a ratiometric fluorescence sensing platform for the precise and quantitative detection of Al3+ ions and pH values, and as optical nanoprobes for cellular imaging.
Highly photoluminescent C-dots with a photoluminescence quantum yield of 26% have been prepared in one step via hydrothermal treatment of orange juice.334 Due to their high photostability and low toxicity, these C-dots were shown to be excellent probes for cellular imaging. A green and sustainable strategy for synthesizing nitrogen-doped C-dots has been reported via hydrothermal treatment of willow leaves.335 The supernatant exhibited strong blue fluorescence under UV radiation, and could be directly used as a fluorescent ink. The solid product obtained via pyrolysis showed excellent electrocatalytic activity for a highly efficient oxygen reduction reaction with great stability, in addition to methanol/CO tolerance that was superior to a commercial Pt/C catalyst.
A simple, economical, and green method for the preparation of water-soluble, fluorescent carbon NPs (CPs) has been reported with a quantum yield of approximately 6.9% via a hydrothermal process using inexpensive waste from pomelo peel as a carbon source.336 The use of such CPs as probes for a fluorescent Hg2+ detection application was explored, which was based on the Hg2+-induced fluorescence quenching of CPs; the sensing system exhibited excellent sensitivity and selectivity toward Hg2+, with a detection limit as low as 0.23 nM. In another greener and low-cost study, water-soluble fluorescent C-dots were prepared under hydrothermal conditions, with Jinhua bergamot plant as a carbon source.337 The as-synthesized C-dots have a better stability and relatively high photoluminescence with a quantum yield of 50.78%, along with a fluorescence lifetime of ca. 3.84 ns. These C-dots can be used for the sensitive and selective fluorescence detection of Hg2+ and Fe3+.
Mewada et al.338 reported the use of an economical, plant-based method for the production of luminescent water-soluble C-dots, using the peel extract of an Indian water plant (Trapa bispinosa) without adding any external oxidizing agent at 90 °C. C-dots (approximately 5–10 nm) were found in the solution with a prominent green fluorescence under UV-light (λex = 365 nm). The UV-vis spectra recorded at different time intervals (30–120 min) displayed the signature absorption of C-dots between 400 and 600 nm. Fluorescence spectra of the dispersion after 120 min of synthesis exhibited characteristic emission peaks of C-dots when excited at 350, 400, 450, and 500 nm. The C-dot structure was found to be turbostratic when evaluated using X-ray diffraction (XRD). C-dots synthesized by this method were found to be exceptionally biocompatible against MDCK cells.
Liu et al.339 synthesized high quantum yield carbon quantum dots (CQDs) via a green hydrothermal method using bamboo leaves. Branched polyethylenimine (BPEI)-capped CQDs (BPEI-CQDs) were prepared by coating the CQDs with BPEI via electrostatic adsorption; they were employed as fluorescent probes for sensitive and selective Cu2+ detection. Experimental results showed that the synthesized CQDs had an average diameter of 3.6 nm, with a narrow size distribution. The biomass-based CQDs offered a high quantum yield of 7.1%. The BPEI-CQD-based sensing system rendered a simple, reliable, and sensitive Cu2+ detection with a detection limit as low as 115 nM and a dynamic range from 0.333 to 66.6 μM. In addition, the BPEI-CQDs were successfully used to detect Cu2+ in river water, demonstrating their excellent selectivity and great potential for analysis of environmental water samples.
Xu et al.340 developed a green synthesis method for water-soluble and well-dispersed fluorescent C-dots via a one-step hydrothermal treatment of potatoes. The as-prepared C-dots exhibited a strong blue fluorescence, with a quantum yield of up to 15%. They explored the use of these C-dots as novel sensing probes for the label-free, sensitive, and selective detection of Fe3+ with a detection limit as low as 0.025 μmol L−1, and different concentrations corresponding to different sensitivities.340
A facile one-pot synthesis of fluorescent C-dots from orange waste peels has been developed via a hydrothermal carbonization method at a mild temperature (180 °C).341 The prepared hydrothermal carbons were amorphous in nature, and clusters of polyaromatic hydrocarbons included a large quantity of oxygen functional groups. A composite of C-dots with ZnO was used as a superior photocatalyst for the degradation of naphthol blue-black Azo dye under UV irradiation. In another study, Park et al.342 developed a simple approach for the large-scale synthesis of water-soluble green carbon nanodots (G-dots) from a variety of food waste-derived sources; ∼120 grams of G-dots per 100 kilograms of food waste were synthesized using this simple and environment-friendly synthesis approach. The G-dots exhibited a high degree of solubility in water because of the abundant oxygen-containing functional groups on their surface. The narrow band of photoluminescence emission (approximately 400–470 nm) confirmed that the size of the G-dots was small (∼4 nm) because of a similar quantum effect and emission trap on the surfaces. The G-dots had excellent photostability and their photoluminescence intensity slowly decreased (∼8%) under continuous excitation with a Xe lamp for 10 days. A cell viability assay was conducted to assess the cytotoxicity effects of the G-dots on cells by introducing concentrations of G-dots, up to 2 mg mL−1 for 24 h. The high photostability and low cytotoxicity exhibited by these G-dots render them excellent probes for in vitro bio-imaging.
3.4. Exosome-like NPs
Communication between cells in multicellular organisms is an undeniable phenomenon. Exosomes are known to play a role in cell–cell communication and have, therefore, become a subject of increasing interest. Exosomes are nanosized microvesicles released from a variety of cells that can carry a cargo of proteins, lipids, mRNAs, and/or microRNAs, and transfer their cargo to recipient cells, thus, serving as extracellular messengers to mediate cell–cell communication. Recent investigations have suggested that nanosized particles from plant cells are exosome-like. In plants, exosomes derived from multivesicular bodies may respond to plant pathogens as a means to regulate the innate immunity of plants. Ju et al.142 reported that the cells targeted by grape exosome-like NPs (GELNs) are intestinal stem cells, whose responses motivate GELN-mediated intestinal tissue remodeling and protection against dextran sulfate sodium (DSS)-induced colitis. This finding is further supported by the fact that co-culturing of crypt or sorted Lgr5+ stem cells with GELNs distinctly improved organoid formation. GELN lipids play a role in the induction of Lgr5+ stem cells, and the liposome-like NPs assembled with lipids from GELNs are required for in vivo targeting of intestinal stem cells. Blocking β-catenin-mediated signaling pathways of GELN recipient cells attenuates the production of Lgr5+ stem cells. Thus, GELNs not only modulate intestinal tissue renewal protocols, but can also participate in their remodeling in response to pathological triggers.Wang et al.143 illustrated that grapefruit-derived nanovesicles (GDNs) were selectively taken up by intestinal macrophages and ameliorated DSS-induced mouse colitis. They demonstrated the development of GDNs for oral delivery of methotrexate to attenuate inflammatory responses in human diseases. Furthermore, exosome-like NPs, isolated and characterized from four edible plants, were reported to contain proteins, lipids, and microRNA. The EPDENs were taken up by intestinal macrophages and stem cells, and the results generated from EPDEN-transfected macrophages indicated that ginger EPDENs preferentially induced the expression of the anti-oxidation gene, heme oxygenase-1, and the anti-inflammatory cytokine, IL-10; whereas, grapefruit, ginger, and carrot EPDENs promoted the activation of nuclear factors (erythroid-derived 2). Furthermore, analysis of the intestines of canonical Wnt-reporter mice revealed that the numbers of β-galactosidase+ (β-Gal) intestinal crypts were increased, suggesting that the EPDEN treatment of mice led to Wnt-mediated activation of the TCF4 transcription machinery in the crypts. These findings suggested a role of EPDEN-mediated interspecies communication by inducing expression of genes for anti-inflammation cytokines, anti-oxidation, and activation of Wnt signaling, which are crucial for maintaining intestinal homeostasis.
3.5. Adhesive NPs
Surface adhesion in nature is not an unknown phenomenon in the literature and several researchers have focused on this area over the past few years as they gear up to understand the chemical aspects of adhesion. While scientists have succeeded in determining some of the molecular structures present in the adhesives secreted by surface climbing or surface affixing biological systems, such as mussels and barnacles, the fundamental underlying adhesion mechanisms are still unknown (Fig. 13). | ||
| Fig. 13 Adhesive NPs – leaves of Sundew (left picture) are covered by small tentacles that generate the adhesive. | ||
Studies have revealed that the natural biological systems utilize NPs to increase the surface adhesion.343 Although, many advances have been made in the examination of micro- to nanoscale attachment mechanisms in animals, little attention has been paid to the detection of a similar phenomenon in plants. Lenaghan and Zhang344 have explained that surfaces where ivy is attached had the presence of uniform NPs that were hypothesized to contribute to its amazing fastening strength. This persuasively illustrates that NPs are related to the adhesive forming natural nanocomposite and highlights the importance of plant adhesion research for bio-inspiration to design strategies for novel nano-scale attachment. Huang et al.145 concluded that the ivy NPs have the ability of preserving their UV protective capability in a wide range of temperatures and pH values, thus offering a potential alternative to replace the existing metal oxide NPs in sunscreen applications.
Xia et al.144 examined the nanostructures formed by the soluble and insoluble parts of the sticky excretion from the mucilaginous rhizome of Dioscorea opposita and evaluated their cellular response. They found that the soluble extract of the excretion is able to shape a nanofibrillar scaffold made of uniform nanofibers, ∼10 nm in size, with a typical pore size of ∼40 nm, while nanofibers shaped by the insoluble extract did not have a specific structure. On the other hand, the nanofibrillar scaffold formed from the soluble extract provided an excellent platform for HeLa cell attachment and growth, and to a lesser degree for MC3T3 cells, while nanofibers from the insoluble extract revealed no cell attachment and growth. The nanofibrillar scaffold created from the Dioscorea opposita extract and its ability to sustain the attachment of specific cell types demonstrated the potential for this natural nanomaterial in tissue engineering applications.
The diverse range of natural nanocomposites and their utility for tissue engineering has become an active topic in biomaterial research. Zhang et al.345 investigated a nanofiber- and NP-based nanocomposite secreted from an insect-capturing plant, the Sundew, for cell attachment; the adhesive nanocomposite has a high biocompatibility and is ready for use with minimal preparation. Atomic force microscopy (AFM) examination of the adhesive from three species of Sundew showed the existence of a nanofiber network and NPs, in various sizes. AFM and light microscopy confirmed that a pattern of nanofibers corresponded to the Alcian Blue staining for polysaccharides. TEM identified a low abundance of NPs in different patterns after AFM observations. Furthermore, the presence of Ca, Mg, and Cl (common components of biological salts) was revealed by EDS. Assessment of the material features of the adhesive showed a high viscoelasticity from the liquid adhesive, with reduced elasticity observed in the dried form. At the same time, they illustrated that the ability of PC12 neuron-like cells to attach and grow on the network of nanofibers, created from the dried adhesive, can be useful in tissue engineering and other biomedical applications.
Yunnan baiyao, a traditional Chinese herbal medicine that has been used to treat wounds for centuries, was examined via AFM by Lenaghan et al.346 which revealed the presence of uniform nanofibers in a relatively high abundance in the solution form; fibers were typically 25.1 nm in diameter and ranged in length from 86–726 nm. The unique structural and adhesive properties of nanofibers may play a vital role in platelet aggregation, leading to clotting, and the sealing of wounds.
3.6. Silica NPs
Elemental silicon has a wide range of traditional applications in metallurgy and in the semiconductor industry.146 Nanostructured silicon materials are promising for a range of new technologies, such as nanoelectronics, photonics, biotechnology, energy harvesting, and energy storage (Fig. 14). Silica NPs have varied and significant applications and can be produced in several forms, including fumed silica, colloidal silica, silica gel, and silica aerogel. Among the available agricultural bio-resources, rice husk is considered to be a cost-effective and non-metallic bio-precursor for synthesizing biogenic silica NPs.Rice (Oryza sativa) is the second-largest produced crop species worldwide (7.0 × 108 metric tons per year). Rice husks (RHs) and sugarcane bagasse are two of the highest-volume agricultural process residues. For every five tons of rice harvested, one ton of husk is produced, amounting to 1.2 × 108 tons of RHs per year across the globe. This enormous amount of waste by-product is an environmental nuisance and developing uses for these waste resources is associated with the global shift toward sustainable method development. However, current applications for the RHs have been limited to those with a low added value, such as fertilizer additives, fuels, and land-fill or paving materials. The discovery pertaining to the recovery of high-value materials from RHs is desirable. RHs burn in air to form rice husk ash, and in this process, the organic matter decomposes offering silica as the major remnant. Surprisingly, silica obtained in this manner is relatively pure and accounts for as much as approximately 20% of the dry weight of the RHs. Moreover, the silica within the RHs naturally exists in the form of NPs. As a living plant, rice absorbs silica in the form of silicic acid from the soil, and the silica accumulates around cellulose micro-compartments. Therefore, RHs are a natural reservoir for nanostructured silica and its derivatives146 (Fig. 15).
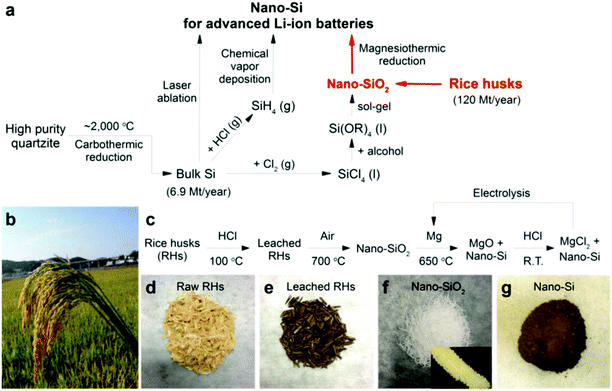 | ||
| Fig. 15 (a) A possibly low-cost, energy-efficient, green, and large scale production of nano-Si from RHs. Methods for producing nano-Si. Si(OR)4 denotes silicon alkoxide. (b) A panicle of ripe rice at a rice farm in China (photographed by Huo, K.). (c) Flow chart of the process for recovering SiNPs from RHs. R.T. denotes room temperature. d–g, Optical images of the intermediate substances. The inset of (f) shows an optical microscopy image (0.9 mm 3 0.6 mm) of one piece of heat treated RH. Reproduced with permission from ref. 146. | ||
Athinarayanan et al.128 synthesized irregular biogenic silica NPs (approximately 10–30 nm) using rice husk as a precursor under pressurized conditions; the acid pretreatment of rice husk helps remove the inorganic impurities and induce the hydrolysis of organic substances. Residues from the acid pretreatment are calcinated at different temperatures for one hour; the produced biogenic silica NPs can be used in bone tissue engineering.128
Biogenic silica NPs (∼25–30 nm) have been synthesized from RHs348 and their characterization revealed that they comprise smaller primary particles (∼4.2 nm in diameter), and their clustering led to a porous structure with a surface area of 164 m2 g−1. Under controlled-melting conditions catalyzed by K+, these silica NP clusters gradually fused to form semi-crystalline porous silica frameworks with a tunable pore size and structural integrity. Chen et al.349 have developed an approach for the comprehensive utilization of RHs to obtain both lignocellulose and high quality porous silica NPs from RHs; most of the lignocellulose in RHs was first extracted by dissolution in ionic liquids, and subsequently separated and collected. The remaining RH residue following extraction, containing a high concentration of silica, was thermally treated to synthesize amorphous porous silica NPs with a high purity and surface area. It was found that during the extraction of lignocellulose using ionic liquids, some metal cations (e.g., K+) which generated a negative effect for the synthesis of silica, could be removed simultaneously thus generating a synergy for this comprehensive approach to make complete utilization of the RH biomass.
Liu et al.146 reported that pure silica NPs could be derived directly from RHs, an abundant agricultural byproduct produced at a rate of 1.2 × 108 tons per year, with a conversion yield as high as 5% by mass. Because of their small size (∼10–40 nm) and porous nature, these recovered silica NPs exhibited a high performance as Li-ion battery anodes, with a high reversible capacity (2790 mA h g−1, seven times greater than graphite anodes) and a long cycle life (86% capacity retention over 300 cycles). Similarly, Xing et al.350 demonstrated a scalable synthetic approach for the transformation of RH into highly valuable porous silicon using a magnesiothermic reaction; synthesized porous silicon, with a high porosity (0.62 cm3 g−1) and a large specific surface area (150.1 m2 g−1), is a promising material for lithium-ion battery applications. As an anode material, the porous silicon retained a considerably high reversible capacity of 1220.2 mA h g−1 at a specific discharge charge current of 1000 mA g−1 after 100 cycles.
Silica NPs from RH ash have been synthesized at room temperature using a high-energy planetary ball mill; the spherical nano-sized amorphous silica particles were formed after about 6 h of ball milling351 and the average particle size was approximately 70 nm, which decreased with increasing the ball milling time or the mill's rotational speed. The as-synthesized silica NPs were subsequently employed as drug carriers for the in vitro release behavior of Penicillin-G in simulated body fluid. Furthermore, Espíndola-Gonzalez et al.147 reported the synthesis of silica oxide NPs from RH, sugar cane bagasse, and coffee husk, by employing vermicompost with annelids (Eisenia foetida); the generated product (humus) was calcinated and extracted to recover the crystalline NPs.
3.7. Lipid-based NPs
 | ||
| Fig. 16 Cardanol as a renewable resource for diverse applications reproduced with permission from the Royal Society of Chemistry.149 | ||
Cardanol, a biobased lipid-mixture obtained from the plant Anacardium occidentale L., is a renewable raw material derived from a byproduct of the cashew nut processing industry. This bio-based lipid-mixture is a rich mixture of non-isoprenoic phenolic compounds, which is a valuable raw material for generating several soft nanomaterials such as nanofibers, nanotubes, gels and surfactants which may then serve as templates for the synthesis of additional nanomaterials149 (Fig. 17).
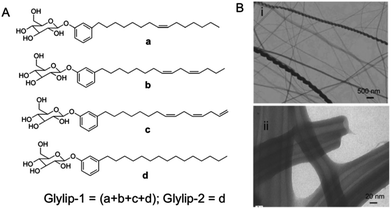 | ||
| Fig. 17 (A) Chemical structures of cardanol glycolipids, and (B) their self-assembly to form (i) helical fibers and (ii) nanotubes. Reproduced from ref. 149 with permission from the Royal Society of Chemistry. | ||
4. Challenges and future scope
The use of plants in green nanotechnology and nanobiotechnology is developing rapidly and plant-derived nanostructures have found numerous applications in diverse fields including photocatalysis, solar cell devices, biosensors, catalysis, biomedicine, electronics, sensing, photonics, environmental clean-up, imaging, bio-labeling, drug and gene delivery, and biomaterials. The non-toxic and biocompatible properties of these nanostructures enable their applications in biomedical sciences such as tissue engineering as well as in pharmaceutical industries.347,352–356There has been a huge surge of interest in the use of biomass as a renewable source of energy and in materials; cellulose, as a nanostructured material in the form of nanocellulose is one, among others. Chemically, it mainly consists of cellulose nanocrystals or mechanically extracted NPs (micro-fibrillated cellulose). On this related theme, several explorations have been conducted for the production of plant-derived nanostructures on a large scale exemplified in the production of starch nanocrystals, curcumin NPs and other nanocellulose-based materials. The ongoing investigations in this area would promote the development of greener methods to generate nanostructures of various shapes and sizes, from renewable and biodegradable precursor chemicals, thus precluding the use of hazardous organic solvents, and associated by-products, which often raise environmental concerns. Although there is a significant benefit in developing non-toxic, inexpensive, and eco-friendly processes in this context, several important challenges and technical problems need to be circumvented. As an example, in phyto-formulation studies, researchers have focused their attention on developing nano dosage forms, including liposomes, proliposomes, solid lipid NPs, nano-emulsions, protein-based and lipid-based drug delivery systems etc. In this domain, bioavailability, enhancement of solubility and toxicity prevention, enhancement of pharmacological activity and stability, improving tissue macrophage distribution, sustained delivery, and avoidance of physical and chemical degradation are some of the important prerequisites. It is crucial to discuss the issue of large-scale production of nanostructures which governs their practical applications as commercial products.
5. Conclusion
The development of biomimetic and bio-inspired approaches to nanostructures is one of the most promising scientific and technological challenges in the coming years for the development of advanced technologies, namely bio-inspired materials and systems, adaptive materials, hierarchically structured materials, 3D composites, and nanomaterials that are compatible with ecological requirements. Bio-inspired and selective multi-functional materials, with associated applications (such as separation, adsorption, catalysis, imaging, biosensing, sensing, and multi-therapy), will undoubtedly be emphasized in future research endeavors for the sustainable use of renewable resources. Greener nanofabrication has been actively pursued in recent years to meet the need for large quantities of highly purified, structurally well-defined, and precisely functionalized nanomaterials. In the present review, an overview of natural nanomaterials originating from live plants or plant-derived materials, and a discussion of their potential applications in diverse disciplines including biomedical applications is presented. Despite the progress achieved to date, considerable challenges exist that must be addressed to obtain optimal performance and derive maximum benefits from the complete and exhaustive use of these plant-based green nano-manufacturing systems.Disclaimer
The U.S. Environmental Protection Agency (EPA), through its Office of Research and Development, partially funded and collaborated in the research described herein. It has been subjected to the Agency's administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the Agency, therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.List of abbreviations
| AFM | Atomic force microscopy |
| BPEI-CQDs | Branched polyethyleneamine-capped carbon quantum dots |
| CBPs | Cranberry Procyanidins |
| CNSL | Cashew nut shell liquid |
| C-dots | Carbon nanodots |
| CNTs | Carbon nanotubes |
| CPs | Carbon nanoparticles |
| CQDs | Carbon quantum dots |
| CMCS | Carboxymethyl chitosan |
| CVD | Chemical vapor deposition |
| DIM | 3,3′-Diindolylmethane |
| EDS | Energy dispersive spectroscopy |
| EOs | Essential oils |
| GI | Gastrointestinal |
| GELNs | Grape exosome-like NPs |
| GDNs | Grapefruit-derived nanovesicles |
| G-dots | Green carbon nanodots |
| 5-FU | 5-Fluro uracil |
| FDA | Food and drug administration |
| FTIR | Fourier transform infrared |
| I3C | Indole-3-carbinol |
| Fe | Iron |
| KET | Ketoprofen |
| MB | Methylene blue |
| MW | Microwave |
| MWt | Molecular weight |
| MWCNTs | Multi-walled carbon nanotubes |
| NPs | Nanoparticles |
| Pd | Palladium |
| PBS | Phosphate buffered saline |
| PCR | Polymerase chain reaction |
| QY | Quantum yield |
| RA | Retinoic acid |
| RHs | Rice husks |
| SEM | Scanning electron microscopy |
| SWNT | Single-wall nanotubes |
| SPI | Soy protein isolate |
| TEMPO | 2,2,6,6-Tetramethylpi-peridine-1-oxyl |
| 3D | Three-dimensional |
| TOC | α-Tocopherol |
| TEM | Transmission electron microscopy |
| UV | Ultra violet |
| VLS | Vapor–liquid–solid |
| VD3 | Vitamin D3 |
| wsCNOs | Water-soluble carbon nano-onions |
| XRD | X-ray diffraction |
| IBU | Zein/Ibuprofen |
| ZN | Zein NPs |
References
- A. Hoffman, G. Mills, H. Yee and M. Hoffmann, J. Phys. Chem., 1992, 96, 5546–5552 CrossRef CAS.
- G. Schmid, Chem. Rev., 1992, 92, 1709–1727 CrossRef CAS.
- V. Colvin, M. Schlamp and A. Alivisatos, Nature, 1994, 370, 354–357 CrossRef CAS.
- Y. Wang and N. Herron, J. Phys. Chem., 1991, 95, 525–532 CrossRef CAS.
- H. S. Mansur, F. Grieser, M. S. Marychurch, S. Biggs, R. S. Urquhart and D. N. Furlong, J. Chem. Soc., Faraday Trans., 1995, 91, 665–672 RSC.
- Y. Wang, Acc. Chem. Res., 1991, 24, 133–139 CrossRef CAS.
- A. Yoffe, Adv. Phys., 1993, 42, 173–262 CrossRef CAS.
- M. M. Azmi, Z. Tehrani, R. Lewis, K.-A. Walker, D. Jones, D. Daniels, S. Doak and O. Guy, Biosens. Bioelectron., 2014, 52, 216–224 CrossRef PubMed.
- D. Pan, L. Mi, Q. Huang, J. Hu and C. Fan, Integr. Biol., 2012, 4, 1155–1163 RSC.
- J. Yao, M. Yang and Y. Duan, Chem. Rev., 2014, 114, 6130–6178 CrossRef CAS PubMed.
- J. Virkutyte and R. S. Varma, Chem. Sci., 2011, 2, 837–846 RSC.
- S. Shukla, C. Dickmeis, A. Nagarajan, R. Fischer, U. Commandeur and N. Steinmetz, Biomater. Sci., 2014, 2, 784–797 RSC.
- A. Kumar and V. Kumar, Chem. Rev., 2014, 114, 7044–7078 CrossRef CAS PubMed.
- K. Ashtari, J. Fasihi, N. Mollania and K. Khajeh, Mater. Res. Bull., 2014, 50, 348–353 Search PubMed.
- S. S. Behrens, Encyclopedia of Inorganic and Bioinorganic Chemistry, Wiley, 2013 Search PubMed.
- K. Cung, B. J. Han, T. D. Nguyen, S. Mao, Y.-W. Yeh, S. Xu, R. R. Naik, G. Poirier, N. Yao and P. K. Purohit, Nano Lett., 2013, 13, 6197–6202 CrossRef CAS PubMed.
- L. Gao and N. Ma, ACS Nano, 2011, 6, 689–695 CrossRef PubMed.
- W. Gao, X. Feng, A. Pei, C. R. Kane, R. Tam, C. Hennessy and J. Wang, Nano Lett., 2013, 14, 305–310 Search PubMed.
- A. Kumar and B. Singh, RSC Adv., 2012, 2, 9079–9090 RSC.
- Z. Tong, Y. Jiang, D. Yang, J. Shi, S. Zhang, C. Liu and Z. Jiang, RSC Adv., 2014, 4, 12388–12403 RSC.
- R. Govaerts, Taxon, 2001, 1085–1090 CrossRef.
- S. Iravani, Green Chem., 2011, 13, 2638–2650 RSC.
- S. Iravani, H. Korbekandi, S. V. Mirmohammadi and B. Zolfaghari, Results Pharma. Sci., 2014, 9, 385–406 CAS.
- S. Iravani and B. Zolfaghari, BioMed. Res. Int., 2013, 2013, 5, DOI:10.1155/2013/639725.
- H. Korbekandi, Z. Ashari, S. Iravani and S. Abbasi, Iran. J. Pharm. Res., 2013, 12, 289–298 CAS.
- H. Korbekandi and S. Iravani, in Green biosynthesis of nanoparticles: mechanisms and applications, ed. M. Rai and C. Posten, CABI, Wallingford, UK, 2013, pp. 53–60 Search PubMed.
- H. Korbekandi, S. Iravani and S. Abbasi, Crit. Rev. Biotechnol., 2009, 29, 279–306 CrossRef CAS PubMed.
- H. Korbekandi, S. Iravani and S. Abbasi, J. Chem. Technol. Biotechnol., 2012, 87, 932–937 CrossRef CAS.
- M. N. Nadagouda, A. B. Castle, R. C. Murdock, S. M. Hussain and R. S. Varma, Green Chem., 2010, 12, 114–122 RSC.
- M. N. Nadagouda, V. Polshettiwar and R. S. Varma, J. Mater. Chem., 2009, 19, 2026–2031 RSC.
- M. N. Nadagouda, T. F. Speth and R. S. Varma, Acc. Chem. Res., 2011, 44, 469–478 CrossRef CAS PubMed.
- M. N. Nadagouda and R. S. Varma, Green Chem., 2008, 10, 859–862 RSC.
- M. N. Nadagouda and R. S. Varma, J. Nanomater., 2008 DOI:10.1155/2008/782358.
- A. Baghizadeh, S. Ranjbar, V. K. Gupta, M. Asif, S. Pourseyedi, M. J. Karimi and R. Mohammadinejad, J. Mol. Liq., 2015, 207, 159–163 CrossRef CAS.
- A. K. Mittal, Y. Chisti and U. C. Banerjee, Biotechnol. Adv., 2013, 31, 346–356 CrossRef CAS PubMed.
- R. Mohammadinejad, S. Pourseyedi, A. Baghizadeh, S. Ranjbar and G. Mansoori, Int. J. Nanosci. Nanotechnol., 2013, 9, 221–226 Search PubMed.
- K. B. A. Ahmed, S. Subramanian, A. Sivasubramanian, G. Veerappan and A. Veerappan, Spectrochim. Acta, Part A, 2014, 130, 54–58 CrossRef PubMed.
- A. Gade, S. Gaikwad, N. Duran and M. Rai, Micron, 2014, 59, 52–59 CrossRef CAS PubMed.
- M. Khan, M. Khan, M. Kuniyil, S. F. Adil, A. Al-Warthan, H. Z. Alkhathlan, W. Tremel, M. N. Tahir and M. R. H. Siddiqui, Dalton Trans., 2014, 43, 9026–9031 RSC.
- D. A. Kumar, V. Palanichamy and S. Roopan, Spectrochim. Acta, Part A, 2014, 127, 168–171 CrossRef CAS PubMed.
- R. Mariselvam, A. J. A. Ranjitsingh, A. Usha Raja Nanthini, K. Kalirajan, C. Padmalatha and P.-M. Selvakumar, Spectrochim. Acta, Part A, 2014, 129, 537–541 CrossRef CAS PubMed.
- C. K. Sathiya and S. Akilandeswari, Spectrochim. Acta, Part A, 2014, 128, 337–341 CrossRef CAS PubMed.
- Q. Sun, X. Caia, J. Li, M. Zheng, Z. Chen and C.-P. Yu, Colloids Surf., A, 2014, 444, 226–231 CrossRef CAS.
- G. Suresh, P. H. Gunasekar, D. Kokila, D. Prabhu, D. Dinesh and N. Ravichandran, et al. , Spectrochim. Acta, Part A, 2014, 127, 61–66 CrossRef CAS PubMed.
- P. Velmurugan, K. Anbalagan, M. Manosathyadevan, K. J. Lee, M. Cho, S. M. Lee, J.-H. Park, S.-G. Oh, K.-S. Bang and B.-T. Oh, Bioprocess Biosyst. Eng., 2014, 37, 1935–1943 CrossRef CAS PubMed.
- S. R. Vijayan, P. Santhiyagu, M. Singamuthu, N. K. Ahila, R. Jayaraman and K. Ethiraj, Sci. World J., 2014, 2014 DOI:10.1155/2014/938272.
- K. Vimala, S. Sundarraj, M. Paulpandi, S. Vengatesan and S. Kannan, Process Biochem., 2014, 49, 160–172 CrossRef CAS.
- R. Yuvakkumar, J. Suresh, A. Joseph Nathanael, M. Sundrarajan and S.-I. Hong, Mater. Sci. Eng., C, 2014, 41, 17–27 CrossRef CAS PubMed.
- C. Coman, L. F. Leopold, O. D. Rugină, L. Barbu-Tudoran, N. Leopold and M. Tofană, et al. , J. Nanopart. Res., 2013, 16, 2158 CrossRef.
- S. Gunalan, R. Sivaraj and R. Venckatesh, Spectrochim. Acta, Part A, 2012, 97, 1140–1144 CrossRef CAS PubMed.
- K. L. Niraimathi, V. Sudha, R. Lavanya and P. Brindha, Colloids Surf., B, 2013, 102, 288–291 CrossRef CAS PubMed.
- S. Sulochana, P. Krishnamoorthy and K. Sivaranjani, J. Pharmacol. Toxicol., 2012, 7, 251–258 CrossRef CAS.
- S. M. Roopan, A. Bharathi, R. Kumar, V. G. Khanna and A. Prabhakarn, Colloids Surf., B, 2012, 92, 209–212 CrossRef CAS PubMed.
- A. J. Kora and J. Arunachala, J. Nanomater., 2012, 2012 DOI:10.1155/2012/869765.
- S. S. Shankar, A. Rai, A. Ahmad and M. Sastry, J. Colloid Interface Sci., 2004, 275, 496–502 CrossRef CAS PubMed.
- A. Thirumurugan, G. J. Jiflin, G. Rajagomathi, N. A. Tomy, S. Ramachandran and R. Jaiganesh, Int. J. Biol. Technol., 2010, 1, 75–77 CAS.
- S. Aswathy Aromal and D. Philip, Physica E, 2012, 44, 1329–1334 CrossRef.
- S. Harne, A. Sharma, M. Dhaygude, S. Joglekar, K. Kodam and M. Hudlikar, Colloids Surf., B, 2012, 95, 284–288 CrossRef CAS PubMed.
- S. K. Boruah, P. K. Boruah, P. Sarma, C. Medhi and O.-K. Medhi, Adv. Mater. Lett., 2012, 3, 481–486 CAS.
- D. Jain, H. K. Daima, S. Kachhwaha and S.-L. Kothari, Dig. J. Nanomater. Bios, 2009, 4, 557–563 Search PubMed.
- N. Mude, A. Ingle, A. Gade and M. Rai, J. Plant Biochem. Biotechnol., 2009, 18, 83–86 CrossRef CAS.
- M. Sathishkumar, K. Sneha, S. K. In, J. Mao, S. J. Tripathy and Y. S. Yun, J. Hazard. Mater., 2009, 171, 400–404 CrossRef CAS PubMed.
- M. Sathishkumar, K. Sneha, S. W. Won, C.-W. Cho, S. Kim and Y. S. Yun, Colloids Surf., B, 2009, 73, 332–338 CrossRef CAS PubMed.
- Y. Xin, L. Qingbiao, W. Huixuan, H. Jiale, L. Liqin and W. Wenta, et al. , J. Nanopart. Res., 2010, 12, 1589–1598 CrossRef.
- K. Satyavani, T. Ramanathan and S. Gurudeeban, J. Nanosci. Nanotechnol., 2011, 1, 95–101 CrossRef.
- M. Vanaja and G. Annadurai, Appl. Nanosci., 2013, 3, 217–223 CrossRef CAS.
- D. Gnanasangeetha and D. SaralaThambavani, Res. J. Mater. Sci., 2013, 1, 1–8 Search PubMed.
- A. K. Jha and K. Prasad, Int. J. Green Nanotechnol., 2010, 1(2), 110–117 CrossRef.
- A. K. Jha, K. Prasad, K. Prasad and A. Kulkarni, Colloids Surf., B, 2009, 73, 219–223 CrossRef CAS PubMed.
- J. Kesharwani, K. Y. Yoon, J. Hwang and M. Rai, J. Bionanosci., 2009, 3, 1–6 Search PubMed.
- N. Ahmad, S. Sharma, V. N. Singh, S. F. Shamsi, A. Fatma and B. R. Mehta, Biotechnol. Res. Int., 2011, 2011 DOI:10.4061/2011/454090.
- J. Y. Song, E. Y. Kwon and B. S. Kim, Bioprocess Biosyst. Eng., 2010, 33, 159–164 CrossRef CAS PubMed.
- S. Ghosh, S. Patil, M. Ahire, R. Kitture, S. Kale and K. Pardesi, Int. J. Nanomed., 2012, 7, 483–496 CAS.
- R. U. Maheswari, A. L. Prabha, V. Nandagopalan and V. Anburaja, IOSR J. Pharm. Biol. Sci., 2012, 1, 38–42 Search PubMed.
- G. Rajakumar, A. A. Rahuman, B. Priyamvada, V. G. Khanna, D. K. Kumar and P.-J. Sujin, Mater. Lett., 2012, 68, 115–117 CrossRef CAS.
- G. Gnanajobitha, G. Annadurai and C. Kannan, Int. J. Pharm. Sci. Res., 2012, 3, 323–330 CAS.
- M. Dubey, S. Bhadauria and B. Kushwah, Dig. J. Nanomater. Biostruct., 2009, 4, 537–543 Search PubMed.
- M. Valodkar, R. N. Jadeja, M. C. Thounaojam, R. V. Devkar and S. Thakore, Mater. Chem. Phys., 2011, 128, 83–89 CrossRef CAS.
- L. Marchiol, A. Mattiello, F. Pošćić, C. Giordano and R. Musetti, Nanoscale Res. Lett., 2014, 9, 101 CrossRef PubMed.
- L. Jia, Q. Zhang, Q. Li and H. Song, Nanotech, 2009, 20, 385601 CrossRef PubMed.
- R. K. Petla, S. Vivekanandhan, M. Misra, A. K. Mohanty and N. Satyanarayana, J. Biomater. Nanobiotechnol., 2012, 3, 14–19 CrossRef CAS.
- S. Dinesh, S. Karthikeyan and P. Arumugam, Arch. Appl. Sci. Res., 2012, 4, 178–187 Search PubMed.
- M. R. Bindhu and M. Umadevi, Spectrochim. Acta, Part A, 2012, 101, 184–190 CrossRef PubMed.
- N. Sable, S. Gaikwad, S. Bonde, A. Gade and M. M. Rai, Nus. Biosci., 2012, 4, 45–49 Search PubMed.
- M. F. Fazaludeena, C. Manickamb, M. A. I. Ashankytyc, M. Q. Ahmedd and Z. B. Quaser, J. Microbiol. Biotechnol. Res., 2012, 2, 23–34 Search PubMed.
- P. Sivakumar, C. Nethradevi and S. Renganathan, Asian J. Pharm. Clin. Res., 2012, 5, 97–101 Search PubMed.
- A.-R. Im, L. Han, E. R. Kim, J. Kim, Y. S. Kim and Y. Park, Phytother. Res., 2012, 26, 1249–1255 CrossRef CAS PubMed.
- J. Y. Song, H.-K. Jang and B. Kim, Process Biochem., 2009, 44, 1133–1138 CrossRef CAS.
- D. M. Ali, N. Thajuddin, K. Jeganathan and M. Gunasekaran, Colloids Surf., B, 2011, 85, 360–365 CrossRef PubMed.
- P. S. Vankar and D. Bajpai, Indian J. Biochem. Biophys., 2010, 47, 157–160 CAS.
- M. EJ and L. Inbathamizh, Asian J. Pharm. Clin. Res., 2012, 5, 159–162 Search PubMed.
- C. Soundarrajan, A. Sankari, P. Dhandapani, S. Maruthamuthu, S. Ravichandran, G. Sozhan and N. Palaniswamy, Bioprocess Biosyst. Eng., 2012, 35, 827–833 CrossRef CAS PubMed.
- C. Ramteke, T. Chakrabarti, B. K. Sarangi and R. Pandey, J. Chem., 2013, 2013 DOI:10.1155/2013/278925.
- V. Vignesh, K. F. Anbarasi, S. Karthikeyeni, G. Sathiyanarayanan, P. Subramanian and R. Thirumurugan, Colloids Surf., A, 2013, 439, 184–192 CrossRef CAS.
- D. A. Kumar, Int. Res. J. Pharm., 2012, 3, 169–173 Search PubMed.
- C. Sundaravadivelan and M. Nalini, Asian Pac. J. Trop. Biomed., 2011, 2012, 1–8 Search PubMed.
- S. S. Shankar, A. Absar and S. Murali, Biotechnol. Prog., 2003, 19, 1627–1631 CrossRef CAS PubMed.
- F. Coccia, L. Tonucci, D. Bosco, M. Bressan and N.-D. Alessandro, Green Chem., 2012, 14, 1073–1078 RSC.
- K. Mallikarjuna, G. R. Dillip, G. Narashima, N. J. Sushma and B. D. P. Raju, Res. J. Nanosci. Nanotechnol., 2012, 2, 17–23 CrossRef.
- K. Mallikarjuna, N. John Sushma, B. V. Subba Reddy, G. Narasimha and B. D. P. Raju, Int. J. Chem. Anal. Sci., 2013, 4, 14–18 CrossRef CAS.
- S. Garg, Int. J. Innovations Biol. Chem. Sci., 2012, 3, 5–10 Search PubMed.
- D. C. Patil, V. S. Patil, P. H. Borase, K. B. Salunke and B. R. Salunkhe, Parasitol. Res., 2012, 110, 1815–1822 CrossRef PubMed.
- S. S. Dash, R. Majumdar, A. K. Sikder, B. G. Bag and B.-K. Patra, Appl. Nanosci., 2014, 4, 485–490 CrossRef CAS.
- A. Nabikhan, K. Kandasamy, A. Raj and N. M. Alikunhi, Colloids Surf., B, 2010, 79, 488–493 CrossRef CAS PubMed.
- M. R. Bindhu and M. Umadevi, Spectrochim. Acta, Part A, 2014, 128, 37–45 CrossRef CAS PubMed.
- M. Amin, F. Anwar, M. R. S. A. Janjua, M. A. Iqbal and U. Rashid, Int. J. Mol. Sci., 2012, 13, 9923–9941 CrossRef CAS PubMed.
- E. C. Njagi, H. Huang, L. Stafford, H. Genuino, H. M. Galindo and J. B. Collins, et al. , Langmuir, 2011, 27, 264–271 CrossRef CAS PubMed.
- R. Deshpande, M. D. Bedre, S. Basavaraja, B. Sawle, S. Y. Manjunath and A. Venkataraman, Colloids Surf., B, 2010, 79, 235–240 CrossRef PubMed.
- S. Yallappa, J. Manjanna, M. A. Sindhe, N. D. Satyanarayan, S. N. Pramod and K. Nagaraja, Spectrochim. Acta, Part A, 2013, 110, 108–115 CrossRef CAS PubMed.
- B. Ankamwar, E–J. Chem., 2010, 7, 1334–1339 CrossRef CAS.
- K. M. Kumar, M. Sinha, B. K. Mandal, A. R. Ghosh, K. S. Kumar and P. S. Reddy, Spectrochim. Acta, Part A, 2012, 91, 228–233 CrossRef PubMed.
- R. Geethalakshmi and D. V. L. Sarada, Int. J. Eng. Sci. Res. Technol., 2010, 2, 970–975 Search PubMed.
- K. Gopalakrishnan, C. Ramesh, V. Ragunathan and M. Thamilselvan, Dig. J. Nanomater. Bios., 2012, 7, 833–839 Search PubMed.
- C. Singh, V. Sharma, K. R. P. Naik, V. Khandelwal and H. Singh, Dig. J. Nanomater. Bios., 2011, 6, 535–542 Search PubMed.
- M. Rai and A. Ingle, Appl. Microbiol. Biotechnol., 2012, 94, 287–293 CrossRef CAS PubMed.
- M. Naderi and A. Danesh-Shahraki, Int. J. Agric. Crop Sci., 2013, 5, 2229–2232 Search PubMed.
- S. Joseph, E. Graber, C. Chia, P. Munroe, S. Donne, T. Thomas, S. Nielsen, C. Marjo, H. Rutlidge and G. Pan, Carbon Manage., 2013, 4, 323–343 CrossRef CAS.
- F. Torney, B. G. Trewyn, V. S. Y. Lin and K. Wang, Nat. Nanotechnol., 2007, 2, 295–300 CrossRef CAS PubMed.
- M. V. Khodakovskaya, K. de Silva, A. S. Biris, E. Dervishi and H. Villagarcia, ACS Nano, 2012, 6, 2128–2135 CrossRef CAS PubMed.
- J. P. Giraldo, M. P. Landry, S. M. Faltermeier, T. P. McNicholas, N. M. Iverson, A. A. Boghossian, N. F. Reuel, A. J. Hilmer, F. Sen and J. A. Brew, Nat. Mater., 2014, 13, 400–408 CrossRef CAS PubMed.
- S. Chandra, S. Pradhan, S. Mitra, P. Patra, A. Bhattacharya, P. Pramanik and A. Goswami, Nanoscale, 2014, 6, 3647–3655 RSC.
- S. Yi, Y. Wang, Y. Huang, L. Xia, L. Sun, S. C. Lenaghan and M. Zhang, J. Biomed. Nanotechnol., 2014, 10, 1016–1029 CrossRef CAS PubMed.
- S. Daus and T. Heinze, Macromol. Biosci., 2010, 10, 211–220 CrossRef CAS PubMed.
- M.-L. Kung, P.-Y. Lin, C.-W. Hsieh, M.-H. Tai, D.-C. Wu, C.-H. Kuo, S.-L. Hsieh, H.-T. Chen and S. Hsieh, ACS Sustainable Chem. Eng., 2014, 2, 1769–1775 CrossRef CAS.
- A. Johnson-Buck, G. Kim, S. Wang, H. J. Hah and R. Kopelman, Mol. Cryst. Liq. Cryst., 2009, 501, 138–144 CrossRef.
- H. Koga, M. Nogi, N. Komoda, T. T. Nge, T. Sugahara and K. Suganuma, NPG Asia Mater., 2014, 6, e93 CrossRef CAS.
- I. Gilca, V. I. Popa and C. Crestini, Ultrason. Sonochem., 2015, 369–375 CrossRef CAS PubMed.
- J. Athinarayanan, V. S. Periasamy, M. Alhazmi, K. A. Alatiah and A. A. Alshatwi, Ceram. Int., 2015, 41, 275–281 CrossRef CAS.
- V. N. Mehta, S. Jha and S. K. Kailasa, Mater. Sci. Eng., C, 2014, 38, 20–27 CrossRef CAS PubMed.
- H. Yuan, D. Li, Y. Liu, X. Xu and C. Xiong, Analyst, 2015, 140, 1428–1431 RSC.
- N. Reddy, Z. Shi, H. Xu and Y. Yang, J. Biomed. Mater. Res., Part A, 2015, 103, 1653–1658 CrossRef PubMed.
- E.-P. Ng, H. Awala, K.-H. Tan, F. Adam, R. Retoux and S. Mintov, Microporous Mesoporous Mater., 2015, 204, 204–209 CrossRef CAS.
- D. Whitford, Proteins: structure and function, John Wiley & Sons, 2013 Search PubMed.
- H. Xu, Q. Jiang, N. Reddy and Y. Yang, J. Mater. Chem., 2011, 21, 18227–18235 RSC.
- N. Lin and A. Dufresne, Eur. Polym. J., 2014, 59, 302–325 CrossRef CAS.
- S. Karimi, P. M. Tahir, A. Karimi, A. Dufresne and A. Abdulkhani, Carbohydr. Polym., 2014, 101, 878–885 CrossRef CAS PubMed.
- L. Berglund, Cellulose-based nanocomposites, in Natural Fibers, Biopolymers and Biocomposites, ed. A. K. Mohanty, M. Misra and L. T. Drzal, CRC Press, Boca Raton, FL, 2005 Search PubMed.
- E. T. Mickelson, Ph.D. Thesis, Rice University, Houston, TX, 1999 Search PubMed.
- H. Muramatsu, Y. A. Kim, K. S. Yang, R. Cruz-Silva, I. Toda, T. Yamada, M. Terrones, M. Endo, T. Hayashi and H. Saitoh, Small, 2014, 10, 2766–2770 CrossRef CAS PubMed.
- X.-W. Chen, O. Timpe, S. B. Hamid, R. Schlögl and D. S. Su, Carbon, 2009, 47, 340–343 CrossRef CAS.
- J. Zhu, J. Jia, F. L. Kwong, D. H. L. Ng and S. C. Tjong, Biomass Bioenergy, 2012, 36, 12–19 CrossRef CAS.
- S. Ju, J. Mu, T. Dokland, X. Zhuang, Q. Wang, H. Jiang, X. Xiang, Z.-B. Deng, B. Wang and L. Zhang, Mol. Ther., 2013, 21, 1345–1357 CrossRef CAS PubMed.
- B. Wang, X. Zhuang, Z.-B. Deng, H. Jiang, J. Mu, Q. Wang, X. Xiang, H. Guo, L. Zhang and G. Dryden, Mol. Ther., 2014, 22, 522–534 CrossRef CAS PubMed.
- L. Xia, S. C. Lenaghan, A. B. Wills, Y. Chen and M. Zhang, Colloids Surf., B, 2011, 88, 425–431 CrossRef CAS PubMed.
- Y. Huang, S. C. Lenaghan, L. Xia, J. N. Burris, C. N. Stewart Jr. and M. Zhang, J. Nanobiotechnol., 2013, 11 CAS.
- N. Liu, K. Huo, M. T. McDowell, J. Zhao and Y. Cui, Sci. Rep., 2013, 3, 1919 Search PubMed.
- A. Espíndola-Gonzalez, A. Martínez-Hernández, C. Angeles-Chávez, V. Castano and C. Velasco-Santos, Nanoscale Res. Lett., 2010, 5, 1408–1417 CrossRef PubMed.
- C. Voirin, S. Caillol, N. V. Sadavarte, B. V. Tawade, B. Boutevin and P. P. Wadgaonkar, Polym. Chem., 2014, 5, 3142–3162 RSC.
- V. S. Balachandran, S. R. Jadhav, P. K. Vemula and G. John, Chem. Soc. Rev., 2013, 42, 427–438 RSC.
- A.-M. Orecchioni, C. Duclairoir, D. Renard and E. Nakache, J. Nanosci. Nanotechnol., 2006, 6, 3171–3178 CrossRef CAS PubMed.
- N. Reddy and Y. Yang, Trends Biotechnol., 2011, 29, 490–498 CrossRef CAS PubMed.
- S. Podaralla and O. Perumal, AAPS PharmSciTech, 2012, 13, 919–927 CrossRef CAS PubMed.
- B. Zhang, Y. Luo and Q. Wang, Food Chem., 2011, 124, 210–220 CrossRef CAS.
- K. Karthikeyan, V. R. Krishnaswamy, R. Lakra, M. S. Kiran and P. S. Korrapati, J. Mater. Sci.: Mater. Med., 2015, 26, 101 CrossRef CAS PubMed.
- H. Xu, L. Shen, L. Xu and Y. Yang, Biomed. Microdevices, 2015, 17, 8 CrossRef PubMed.
- T. Zou and L. Gu, Mol. Pharm., 2013, 10, 2062–2070 CrossRef CAS PubMed.
- L. Lai and H. Guo, Int. J. Pharm., 2011, 404, 317–323 CrossRef CAS PubMed.
- R. G. Aswathy, B. Sivakumar, D. Brahatheeswaran, T. Fukuda, Y. Yoshida, T. Maekawa and D. S. Kumar, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2012, 3, 025006 CrossRef.
- Y. Zhang, Y. Niu, Y. Luo, M. Ge, T. Yang, L. L. Yu and Q. Wang, Food Chem., 2014, 142, 269–275 CrossRef CAS PubMed.
- S. Lee, N. S. A. Alwahab and Z. M. Moazzam, Int. J. Pharm., 2013, 454, 388–393 CrossRef CAS PubMed.
- J. Chen, J. Zheng, D. J. McClements and H. Xiao, Food Chem., 2014, 158, 466–472 CrossRef CAS PubMed.
- Y. Luo, B. Zhang, M. Whent, L. L. Yu and Q. Wang, Colloids Surf., B, 2011, 85, 145–152 CrossRef CAS PubMed.
- Y. Luo, Z. Teng and Q. Wang, J. Agric. Food Chem., 2012, 60, 836–843 CrossRef CAS PubMed.
- Y. Luo, T. T. Wang, Z. Teng, P. Chen, J. Sun and Q. Wang, Food Chem., 2013, 139, 224–230 CrossRef CAS PubMed.
- Y. Wu, Y. Luo and Q. Wang, LWT–Food Sci. Technol., 2012, 48, 283–290 CrossRef CAS.
- M. C. Regier, J. D. Taylor, T. Borcyk, Y. Yang and A. K. Pannier, J. Nanobiotechnol., 2012, 10, 44 CrossRef CAS PubMed.
- J. Gomez-Estaca, M. Balaguer, R. Gavara and P. Hernandez-Munoz, Food Hydrocolloids, 2012, 28, 82–91 CrossRef CAS.
- Y.-N. Jiang, H.-Y. Mo and D.-G. Yu, Int. J. Pharm., 2012, 438, 232–239 CrossRef CAS PubMed.
- W. Huang, T. Zou, S. Li, J. Jing, X. Xia and X. Liu, AAPS PharmSciTech, 2013, 14, 675–681 CrossRef CAS PubMed.
- L.-J. Sun, P.-Q. Shen and Y.-P. Zhao, Fine Chem., 2011, 3, 014 Search PubMed.
- D. Hu, C. Lin, L. Liu, S. Li and Y. Zhao, J. Food Eng., 2012, 109, 545–552 CrossRef CAS.
- T. Zou, Z. Li, S. S. Percival, S. Bonard and L. Gu, Food Hydrocolloids, 2012, 27, 293–300 CrossRef CAS.
- Q. Zhong and M. Jin, J. Agric. Food Chem., 2009, 57, 3886–3894 CrossRef CAS PubMed.
- I. Ezpeleta, J. M. Irache, S. Stainmesse, C. Chabenat, J. Gueguen, Y. Popineau and A.-M. Orecchioni, Int. J. Pharm., 1996, 131, 191–200 CrossRef CAS.
- M. A. Arangoa, M. A. Campanero, M. J. Renedo, G. Ponchel and J. M. Irache, Pharm. Res., 2001, 18, 1521–1527 CrossRef CAS.
- C. Duclairoir, A. Orecchioni, P. Depraetere and E. Nakache, J. Microencapsulation, 2002, 19, 53–60 CrossRef CAS PubMed.
- H. Kajal and A. Misra, J. Biomed. Nanotechnol., 2011, 7, 211–212 CrossRef CAS PubMed.
- M. Gulfam, J.-e. Kim, J. M. Lee, B. Ku, B. H. Chung and B. G. Chung, Langmuir, 2012, 28, 8216–8223 CrossRef CAS PubMed.
- N. D. Young and A. K. Bharti, Annu. Rev. Plant Biol., 2012, 63, 283–305 CrossRef CAS PubMed.
- Z. M. Gao, L. P. Zhu, X. Q. Yang, X. T. He, J. M. Wang, J. Guo, J. R. Qi, L. J. Wang and S. Yin, Food Funct., 2014, 5, 1286–1293 CAS.
- T. Mirshahi, J. Irache, J. Gueguen and A. Orecchioni, Drug Dev. Ind. Pharm., 1996, 22, 841–846 CrossRef CAS.
- Z. Teng, Y. Luo and Q. Wang, J. Agric. Food Chem., 2012, 60, 2712–2720 CrossRef CAS PubMed.
- T. H. Wegner and E. P. Jones, Nanosci. Technol. Renewable Biomater., 2009, 1, 1–41 Search PubMed.
- A. Bledzki, S. Reihmane and J. Gassan, Polym.-Plast. Technol. Eng., 1998, 37, 451–468 CrossRef CAS.
- A. Mohanty, M. Misra and L. Drzal, J. Polym. Environ., 2002, 10, 19–26 CrossRef CAS.
- L. A. Lucia and O. J. Rojas, The nanoscience and technology of renewable biomaterials, Wiley Online Library, 2009 Search PubMed.
- D. Klemm, F. Kramer, S. Moritz, T. Lindström, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 5438–5466 CrossRef CAS PubMed.
- M. A. S. Azizi Samir, F. Alloin and A. Dufresne, Biomacromolecules, 2005, 6, 612–626 CrossRef PubMed.
- J. F. Beecher, Nat. Nanotechnol., 2007, 2, 466–467 CrossRef CAS PubMed.
- J. Etang Ayuk, A. P. Mathew and K. Oksman, J. Appl. Polym. Sci., 2009, 114, 2723–2730 CrossRef CAS.
- M. J. John and S. Thomas, Carbohydr. Polym., 2008, 71, 343–364 CrossRef CAS.
- S. Kalia, A. Dufresne, B. M. Cherian, B. Kaith, L. Avérous, J. Njuguna and E. Nassiopoulos, Int. J. Polym. Sci., 2011, 2011 DOI:10.1155/2011/837875.
- A. Bledzki and J. Gassan, Prog. Polym. Sci., 1999, 24, 221–274 CrossRef CAS.
- I. Sakurada, Y. Nukushina and T. Ito, J. Polym. Sci., 1962, 57, 651–660 CrossRef CAS.
- H. Wei, K. Rodriguez, S. Renneckar and P. J. Vikesland, Environ. Sci.: Nano, 2014, 1, 302–316 RSC.
- A. Dufresne, Nanocellulose: from nature to high performance tailored materials, Walter de Gruyter, 2012 Search PubMed.
- A. Dufresne, Mater. Today, 2013, 16, 220–227 CrossRef CAS.
- R. J. Moon, McGraw-Hill, Yeabook in Science & Technology, McGraw-Hill, 2008, pp. 225–228 Search PubMed.
- P. Zadorecki and A. J. Michell, Polym. Compos., 1989, 10, 69–77 CrossRef CAS.
- J. Shi, S. Q. Shi, H. M. Barnes and C. U. Pittman Jr., BioResources, 2011, 6, 879–890 CAS.
- A. Chakraborty, M. Sain and M. Kortschot, Holzforschung, 2005, 59, 102–107 CrossRef CAS.
- A. Isogai, J. Wood Sci., 2013, 59, 449–459 CrossRef CAS.
- J. Zhao, W. Zhang, X. Zhang, X. Zhang, C. Lu and Y. Deng, Carbohydr. Polym., 2013, 97, 695–702 CrossRef CAS PubMed.
- E. Dinand, H. Chanzy and M. Vignon, Cellulose, 1996, 3, 183–188 CrossRef CAS.
- M. Sain and A. Bhatnagar, Can. Pat. Appl., CA 2437616, 2003 Search PubMed.
- A. Alemdar and M. Sain, Compos. Sci. Technol., 2008, 68, 557–565 CrossRef CAS.
- A. Dufresne, J. Y. Cavaillé and W. Helbert, Polym. Compos., 1997, 18, 198–210 CrossRef CAS.
- J. Zhang, H. Song, L. Lin, J. Zhuang, C. Pang and S. Liu, Biomass Bioenergy, 2012, 39, 78–83 CrossRef CAS.
- A. Dufresne, D. Dupeyre and M. R. Vignon, J. Appl. Polym. Sci., 2000, 76, 2080–2092 CrossRef CAS.
- X. Cao, Y. Chen, P. R. Chang, M. Stumborg and M. A. Huneault, J. Appl. Polym. Sci., 2008, 109, 3804–3810 CrossRef CAS.
- B. Wang, M. Sain and K. Oksman, Appl. Compos. Mater., 2007, 14, 89–103 CrossRef CAS.
- B. Wang and M. Sain, Polym. Int., 2007, 56, 538–546 CrossRef CAS.
- E. Dinand, H. Chanzy and R. Vignon, Food Hydrocolloids, 1999, 13, 275–283 CrossRef CAS.
- A. Dufresne, J.-Y. Cavaille and M. R. Vignon, J. Appl. Polym. Sci., 1997, 64, 1185–1194 CrossRef CAS.
- C. Goussé, H. Chanzy, M. Cerrada and E. Fleury, Polymer, 2004, 45, 1569–1575 CrossRef.
- Y. Habibi and M. R. Vignon, Cellulose, 2008, 15, 177–185 CrossRef CAS.
- D. Bhattacharya, L. T. Germinario and W. T. Winter, Carbohydr. Polym., 2008, 73, 371–377 CrossRef CAS.
- E. d. M. Teixeira, D. Pasquini, A. A. Curvelo, E. Corradini, M. N. Belgacem and A. Dufresne, Carbohydr. Polym., 2009, 78, 422–431 CrossRef CAS.
- J. Li, X. Wei, Q. Wang, J. Chen, G. Chang, L. Kong, J. Su and Y. Liu, Carbohydr. Polym., 2012, 90, 1609–1613 CrossRef CAS PubMed.
- Y. Lu, L. Weng and X. Cao, Macromol. Biosci., 2005, 5, 1101–1107 CrossRef CAS PubMed.
- X. Cao, Y. Chen, P. Chang, A. Muir and G. Falk, Express Polym. Lett., 2008, 2, 502–510 CrossRef CAS.
- Y. Lu, L. Weng and X. Cao, Carbohydr. Polym., 2006, 63, 198–204 CrossRef CAS.
- Y. Chen, C. Liu, P. R. Chang, D. P. Anderson and M. A. Huneault, Polym. Eng. Sci., 2009, 49, 369–378 CAS.
- Y. Chen, C. Liu, P. R. Chang, X. Cao and D. P. Anderson, Carbohydr. Polym., 2009, 76, 607–615 CrossRef CAS.
- M. Jonoobi, J. Harun, P. M. Tahir, A. Shakeri, S. SaifulAzry and M. D. Makinejad, Mater. Lett., 2011, 65, 1098–1100 CrossRef CAS.
- M. Jonoobi, K. O. Niska, J. Harun and M. Misra, BioResources, 2009, 4, 626–639 CAS.
- M. Joonobi, J. Harun, P. M. Tahir, L. H. Zaini, S. SaifulAzry and M. D. Makinejad, BioResources, 2010, 5, 2556–2566 Search PubMed.
- H. Kargarzadeh, I. Ahmad, I. Abdullah, A. Dufresne, S. Y. Zainudin and R. M. Sheltami, Cellulose, 2012, 19, 855–866 CrossRef CAS.
- L. H. Zaini, M. Jonoobi, P. M. Tahir and S. Karimi, J. Biomater. Nanobiotechnol., 2013, 4, 37–44 CrossRef CAS.
- A. Alemdar and M. Sain, Bioresour. Technol., 2008, 99, 1664–1671 CrossRef CAS PubMed.
- M. Jonoobi, A. Khazaeian, P. M. Tahir, S. S. Azry and K. Oksman, Cellulose, 2011, 18, 1085–1095 CrossRef CAS.
- N. Ngadi and N. S. Lani, Jurnal Teknologi, 2014, 68, 35–39 CrossRef.
- D. Bruce, R. Hobson, J. Farrent and D. Hepworth, Composites, Part A, 2005, 36, 1486–1493 CrossRef.
- J. I. Morán, V. A. Alvarez, V. P. Cyras and A. Vázquez, Cellulose, 2008, 15, 149–159 CrossRef.
- X. Cao, B. Ding, J. Yu and S. S. Al-Deyab, Carbohydr. Polym., 2012, 90, 1075–1080 CrossRef CAS PubMed.
- M. S. Jahan, A. Saeed, Z. He and Y. Ni, Cellulose, 2011, 18, 451–459 CrossRef CAS.
- R. Zuluaga, J.-L. Putaux, A. Restrepo, I. Mondragon and P. Ganán, Cellulose, 2007, 14, 585–592 CrossRef CAS.
- N. Johar, I. Ahmad and A. Dufresne, Ind. Crops Prod., 2012, 37, 93–99 CrossRef CAS.
- L. Ludueña, D. Fasce, V. A. Alvarez and P. M. Stefani, BioResources, 2011, 6, 1440–1453 Search PubMed.
- F. Fahma, S. Iwamoto, N. Hori, T. Iwata and A. Takemura, Cellulose, 2011, 18, 443–450 CrossRef CAS.
- M. Rosa, E. Medeiros, J. Malmonge, K. Gregorski, D. Wood, L. Mattoso, G. Glenn, W. Orts and S. Imam, Carbohydr. Polym., 2010, 81, 83–92 CrossRef CAS.
- R. M. Sheltami, I. Abdullah, I. Ahmad, A. Dufresne and H. Kargarzadeh, Carbohydr. Polym., 2012, 88, 772–779 CrossRef CAS.
- H. El-Saied, A. H. Basta and R. H. Gobran, Polym.-Plast. Technol. Eng., 2004, 43, 797–820 CrossRef CAS.
- E. E. Brown and M.-P. G. Laborie, Biomacromolecules, 2007, 8, 3074–3081 CrossRef CAS PubMed.
- M. Iguchi, S. Yamanaka and A. Budhiono, J. Mater. Sci., 2000, 35, 261–270 CrossRef CAS.
- Y. Dahman, J. Nanosci. Nanotechnol., 2009, 9, 5105–5122 CrossRef CAS PubMed.
- C. J. Grande, F. G. Torres, C. M. Gomez, O. P. Troncoso, J. Canet-Ferrer and J. Martinez-Pastor, Polym. Polym. Compos., 2008, 16, 181–185 CAS.
- S. Ifuku, M. Nogi, K. Abe, K. Handa, F. Nakatsubo and H. Yano, Biomacromolecules, 2007, 8, 1973–1978 CrossRef CAS PubMed.
- H. Maeda, M. Nakajima, T. Hagiwara, T. Sawaguchi and S. Yano, J. Mater. Sci., 2006, 41, 5646–5656 CrossRef CAS.
- A. Nakagaito, S. Iwamoto and H. Yano, Appl. Phys. A, 2005, 80, 93–97 CrossRef CAS.
- M. Nogi, S. Ifuku, K. Abe, K. Handa, A. N. Nakagaito and H. Yano, Appl. Phys. Lett., 2006, 88, 1–3 Search PubMed.
- M. Nogi and H. Yano, Adv. Mater., 2008, 20, 1849–1852 CrossRef CAS.
- H. Yano, J. Sugiyama, A. N. Nakagaito, M. Nogi, T. Matsuura, M. Hikita and K. Handa, Adv. Mater., 2005, 17, 153–155 CrossRef CAS.
- S. Yano, H. Maeda, M. Nakajima, T. Hagiwara and T. Sawaguchi, Cellulose, 2008, 15, 111–120 CrossRef CAS.
- M. A. Hubbe, O. J. Rojas, L. A. Lucia and M. Sain, BioResources, 2008, 3, 929–980 Search PubMed.
- A. Nakagaito and H. Yano, Appl. Phys. A, 2004, 78, 547–552 CrossRef CAS.
- S. Iwamoto, A. Nakagaito, H. Yano and M. Nogi, Appl. Phys. A, 2005, 81, 1109–1112 CrossRef CAS.
- M. E. Malainine, M. Mahrouz and A. Dufresne, Compos. Sci. Technol., 2005, 65, 1520–1526 CrossRef CAS.
- M. Andresen, L.-S. Johansson, B. S. Tanem and P. Stenius, Cellulose, 2006, 13, 665–677 CrossRef CAS.
- M. Andresen and P. Stenius, J. Dispersion Sci. Technol., 2007, 28, 837–844 CrossRef CAS.
- P. Stenstad, M. Andresen, B. S. Tanem and P. Stenius, Cellulose, 2008, 15, 35–45 CrossRef CAS.
- K. Syverud and P. Stenius, Cellulose, 2009, 16, 75–85 CrossRef CAS.
- A. Bhatnagar and M. Sain, J. Reinf. Plast. Compos., 2005, 24, 1259–1268 CrossRef CAS.
- B. Wang and M. Sain, Compos. Sci. Technol., 2007, 67, 2521–2527 CrossRef CAS.
- K. L. Spence, R. A. Venditti, O. J. Rojas, Y. Habibi and J. J. Pawlak, Cellulose, 2011, 18, 1097–1111 CrossRef CAS.
- K. Abe, S. Iwamoto and H. Yano, Biomacromolecules, 2007, 8, 3276–3278 CrossRef CAS PubMed.
- S. Iwamoto, A. Nakagaito and H. Yano, Appl. Phys. A, 2007, 89, 461–466 CrossRef CAS.
- M. Henriksson, L. A. Berglund, P. Isaksson, T. Lindstrom and T. Nishino, Biomacromolecules, 2008, 9, 1579–1585 CrossRef CAS PubMed.
- S. Karimi, A. Dufresne, P. M. Tahir, A. Karimi and A. Abdulkhani, J. Mater. Sci., 2014, 49, 4513–4521 CrossRef CAS.
- B. Wang and M. Sain, BioResources, 2007, 2, 371–388 CAS.
- E. Lasseuguette, D. Roux and Y. Nishiyama, Cellulose, 2008, 15, 425–433 CrossRef CAS.
- T. Saito, M. Hirota, N. Tamura, S. Kimura, H. Fukuzumi, L. Heux and A. Isogai, Biomacromolecules, 2009, 10, 1992–1996 CrossRef CAS PubMed.
- T. Saito and A. Isogai, Carbohydr. Polym., 2005, 61, 183–190 CrossRef CAS.
- T. Saito and A. Isogai, Colloids Surf., A, 2006, 289, 219–225 CrossRef CAS.
- T. Saito and A. Isogai, Ind. Eng. Chem. Res., 2007, 46, 773–780 CrossRef CAS.
- T. Saito, S. Kimura, Y. Nishiyama and A. Isogai, Biomacromolecules, 2007, 8, 2485–2491 CrossRef CAS PubMed.
- T. Saito, Y. Nishiyama, J.-L. Putaux, M. Vignon and A. Isogai, Biomacromolecules, 2006, 7, 1687–1691 CrossRef CAS PubMed.
- A. Isogai, T. Saito and H. Fukuzumi, Nanoscale, 2011, 3, 71–85 RSC.
- F. Jiang and Y.-L. Hsieh, Carbohydr. Polym., 2013, 95, 32–40 CrossRef CAS PubMed.
- M. Henriksson, G. Henriksson, L. Berglund and T. Lindström, Eur. Polym. J., 2007, 43, 3434–3441 CrossRef CAS.
- M. Pääkkö, M. Ankerfors, H. Kosonen, A. Nykänen, S. Ahola, M. Österberg, J. Ruokolainen, J. Laine, P. T. Larsson, O. Ikkala and T. Lindström, Biomacromolecules, 2007, 8, 1934–1941 CrossRef PubMed.
- S. Janardhnan and M. M. Sain, BioResources, 2007, 1, 176–188 Search PubMed.
- I. Siró and D. Plackett, Cellulose, 2010, 17, 459–494 CrossRef.
- M. M. de Souza Lima and R. Borsali, Macromol. Rapid Commun., 2004, 25, 771–787 CrossRef CAS.
- I. M. Saxena and R. M. Brown, Ann. Bot., 2005, 96, 9–21 CrossRef CAS PubMed.
- W. Thielemans, C. R. Warbey and D. A. Walsh, Green Chem., 2009, 11, 531–537 RSC.
- S. Camarero Espinosa, T. Kuhnt, E. J. Foster and C. Weder, Biomacromolecules, 2013, 14, 1223–1230 CrossRef CAS PubMed.
- M. N. Angles and A. Dufresne, Macromolecules, 2000, 33, 8344–8353 CrossRef CAS.
- A. P. Mathew and A. Dufresne, Biomacromolecules, 2002, 3, 1101–1108 CrossRef CAS PubMed.
- A. P. Mathew, W. Thielemans and A. Dufresne, J. Appl. Polym. Sci., 2008, 109, 4065–4074 CrossRef CAS.
- A. J. Carvalho, Starch: major sources, properties and applications as thermoplastic materials, Elsevier, Amsterdam, 2008 Search PubMed.
- P. M. Visakh, P. M. Aji, K. Oksman and S. Thomas, Starch-Based Bionanocomposites: Processing and Properties, in Polysaccharide Building Blocks: A Sustainable Approach to the Development of Renewable Biomaterials, ed. Y. Habibi and L. A. Lucia, John Wiley and Sons, 2012, pp. 287–306 Search PubMed.
- H. Zobel, Starch/Staerke, 1988, 40, 44–50 CrossRef CAS.
- A. Imberty, A. Buléon, V. Tran and S. Péerez, Starch/Staerke, 1991, 43, 375–384 CrossRef CAS.
- F. Xie, E. Pollet, P. J. Halley and L. Avérous, Prog. Polym. Sci., 2013, 38, 1590–1628 CrossRef CAS.
- H. Liu, L. Yu, F. Xie and L. Chen, Carbohydr. Polym., 2006, 65, 357–363 CrossRef CAS.
- F. Xie, L. Yu, B. Su, P. Liu, J. Wang, H. Liu and L. Chen, J. Cereal Sci., 2009, 49, 371–377 CrossRef CAS.
- D. Le Corre, J. Bras and A. Dufresne, Biomacromolecules, 2010, 11, 1139–1153 CrossRef CAS PubMed.
- A. Dufresne, J.-Y. Cavaille and W. Helbert, Macromolecules, 1996, 29, 7624–7626 CrossRef CAS.
- J.-L. Putaux, S. Molina-Boisseau, T. Momaur and A. Dufresne, Biomacromolecules, 2003, 4, 1198–1202 CrossRef CAS PubMed.
- G. Chen, M. Wei, J. Chen, J. Huang, A. Dufresne and P. R. Chang, Polymer, 2008, 49, 1860–1870 CrossRef CAS.
- Y. Chen, X. Cao, P. R. Chang and M. A. Huneault, Carbohydr. Polym., 2008, 73, 8–17 CrossRef CAS.
- H. Zheng, F. Ai, P. R. Chang, J. Huang and A. Dufresne, Polym. Compos., 2009, 30, 474–480 CrossRef CAS.
- N. L. García, L. Ribba, A. Dufresne, M. I. Aranguren and S. Goyanes, Macromol. Mater. Eng., 2009, 294, 169–177 CrossRef.
- H. Namazi and A. Dadkhah, J. Appl. Polym. Sci., 2008, 110, 2405–2412 CrossRef CAS.
- H. Angellier, S. Molina-Boisseau and A. Dufresne, Macromolecules, 2005, 38, 9161–9170 CrossRef CAS.
- F. Fathi, A. Dadkhah and H. Namazi, Int. J. Nanopart., 2014, 7, 37–48 CrossRef CAS.
- Y. Wang and L. Zhang, J. Nanosci. Nanotechnol., 2008, 8, 5831–5838 CrossRef CAS PubMed.
- Y. Xu, E. N. Sismour, C. Grizzard, M. Thomas, D. Pestov, Z. Huba, T. Wang and H. L. Bhardwaj, Cereal Chem., 2014, 91, 383–388 CrossRef CAS.
- H. Angellier, L. Choisnard, S. Molina-Boisseau, P. Ozil and A. Dufresne, Biomacromolecules, 2004, 5, 1545–1551 CrossRef CAS PubMed.
- N. Lin and A. Dufresne, Nanoscale, 2014, 6, 5384–5393 RSC.
- M. Kaushik, K. Basu, C. Benoit, C. M. Cirtiu, H. Vali and A. Moores, J. Am. Chem. Soc., 2015, 137, 6124–6127 CrossRef CAS PubMed.
- J. Qu, C. Luo, Q. Zhang, Q. Cong and X. Yuan, Mater. Sci. Eng., B, 2013, 178, 380–382 CrossRef CAS.
- J. Qu, Q. Zhang, Y. Xia, Q. Cong and C. Luo, Environ. Sci. Pollut. Res. Int., 2015, 22, 1408–1419 CrossRef CAS PubMed.
- P. Roy, A. P. Periasamy, C. Chuang, Y.-R. Liou, Y.-F. Chen, J. Joly, C.-T. Liang and H.-T. Chang, New J. Chem, 2014, 38, 4946–4951 RSC.
- I. Rhee, Y. A. Kim, G.-O. Shin, J. H. Kim and H. Muramatsu, Constr. Build. Mater., 2015, 96, 189–197 CrossRef.
- C. Wang, D. Ma and X. Bao, J. Phys. Chem. C, 2008, 112, 17596–17602 CAS.
- M. Saxena and S. Sarkar, Mater. Express, 2013, 3, 201–209 CrossRef CAS.
- M. Ghosh, S. K. Sonkar, M. Saxena and S. Sarkar, Small, 2011, 7, 3170–3177 CrossRef CAS PubMed.
- S. K. Sonkar, M. Ghosh, M. Roy, A. Begum and S. Sarkar, Mater. Express, 2012, 2, 105–114 CrossRef CAS.
- S. Kumará Sonkar and D. GorakháBabar, Nanoscale, 2012, 4, 7670–7675 RSC.
- C. Journet, M. Picher and V. Jourdain, Nanotechnology, 2012, 23, 142001 CrossRef PubMed.
- M. F. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- B. Goodell, X. Xie, Y. Qian, G. Daniel, M. Peterson and J. Jellison, J. Nanosci. Nanotechnol., 2008, 8, 2472–2474 CrossRef CAS PubMed.
- S. Paul, Thesis, 2013, http://ir.inflibnet.ac.in:8080/jspui/handle/10603/9008 Search PubMed.
- X. Ye, Q. Yang, Y. Zheng, W. Mo, J. Hu and W. Huang, Mater. Res. Bull., 2014, 51, 366–371 CrossRef CAS.
- X. Xie, B. Goodell, Y. Qian, G. Daniel, D. Zhang, D. C. Nagle, M. L. Peterson and J. Jellison, For. Prod. J., 2009, 59, 26–28 CAS.
- J. Zhao, X. Guo, Q. Guo, L. Gu, Y. Guo and F. Feng, Carbon, 2011, 49, 2155–2158 CrossRef CAS.
- L. Dubrovina, O. Naboka, V. Ogenko, P. Gatenholm and P. Enoksson, J. Mater. Sci., 2014, 49, 1144–1149 CrossRef CAS.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- P.-C. Hsu, Z.-Y. Shih, C.-H. Lee and H.-T. Chang, Green Chem., 2012, 14, 917–920 RSC.
- B. De and N. Karak, RSC Adv., 2013, 3, 8286–8290 RSC.
- L. Shi, X. Li, Y. Li, X. Wen, J. Li, M. M. F. Choi, C. Dong and S. Shuang, Sens. Actuators, B, 2015, 210, 533–541 CrossRef CAS.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- S. Gao, Y. Chen, H. Fan, X. Wei, C. Hu, L. Wang and L. Qu, J. Mater. Chem. A, 2014, 2, 6320–6325 CAS.
- W. Lu, X. Qin, S. Liu, G. Chang, Y. Zhang, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Anal. Chem., 2012, 84, 5351–5357 CrossRef CAS PubMed.
- J. Yu, N. Song, Y.-K. Zhang, S.-X. Zhong, A.-J. Wang and J. Chen, Sens. Actuators, B, 2015, 214, 29–35 CrossRef CAS.
- A. Mewada, S. Pandey, S. Shinde, N. Mishra, G. Oza, M. Thakur, M. Sharon and M. Sharon, Mater. Sci. Eng., C, 2013, 33, 2914–2917 CrossRef CAS PubMed.
- Y. Liu, Y. Zhao and Y. Zhang, Sens. Actuators, B, 2014, 196, 647–652 CrossRef CAS.
- J. Xu, Y. Zhou, S. Liu, M. Dong and C. Huang, Anal. Methods, 2014, 6, 2086–2090 RSC.
- A. Prasannan and T. Imae, Ind. Eng. Chem. Res., 2013, 52, 15673–15678 CrossRef CAS.
- S. Y. Park, H. U. Lee, E. S. Park, S. C. Lee, J.-W. Lee, S. W. Jeong, C. H. Kim, Y.-C. Lee, Y. S. Huh and J. Lee, ACS Appl.Mater. Interfaces, 2014, 6, 3365–3370 CAS.
- M. Zhang, M. Liu, S. Bewick and Z. Suo, J. Biomed. Nanotechnol., 2009, 5, 294–299 CrossRef CAS PubMed.
- S. C. Lenaghan and M. Zhang, Plant Sci., 2012, 183, 206–211 CrossRef CAS PubMed.
- M. Zhang, S. C. Lenaghan, L. Xia, L. Dong, W. He, W. R. Henson and X. Fan, J. Nanobiotechnol., 2010, 8, 20 CrossRef PubMed.
- S. C. Lenaghan, L. Xia and M. Zhang, J. Biomed. Nanotechnol., 2009, 5, 472–476 CrossRef CAS PubMed.
- V. H. Le, C. N. H. Thuc and H. H. Thu, Nanoscale Res. Lett., 2013, 8, 58–67 CrossRef PubMed.
- W. Wang, J. C. Martin, X. Fan, A. Han, Z. Luo and L. Sun, ACS Appl. Mater. Interfaces, 2012, 4, 977–981 CAS.
- H. Chen, W. Wang, J. C. Martin, A. J. Oliphant, P. A. Doerr, J. F. Xu, K. M. DeBorn, C. Chen and L. Sun, ACS Sustainable Chem. Eng., 2012, 1, 254–259 CrossRef.
- A. Xing, S. Tian, H. Tang, D. Losic and Z. Bao, RSC Adv., 2013, 3, 10145–10149 RSC.
- M. Salavati-Niasari, J. Javidi and M. Dadkhah, Comb. Chem. High Throughput Screening, 2013, 16, 458–462 CrossRef CAS PubMed.
- S. C. L. J. N. Burris, M. Zhang and C. N. Stewart, J. Nanobiotechnol., 2012, 10, 41 CrossRef PubMed.
- A. M. R. J. Moon, J. Nairn, J. Simonsenf and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941–3994 RSC.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC.
- R. J. W. M.-M. Titirici, N. Brun, V. L. Budarin, D. S. Su, F. del Monte, J. H. Clarkd and M. J. MacLachlang, Chem. Soc. Rev., 2015, 44, 250–290 RSC.
- Y. Luo and Q. Wang, J. Appl. Polym. Sci., 2014, 131 DOI:10.1002/APP.40696.
| This journal is © The Royal Society of Chemistry 2016 |