 Open Access Article
Open Access ArticleA ligand substituted tungsten iodide cluster: luminescence vs. singlet oxygen production†
Lara
Riehl
a,
Alexander
Seyboldt
a,
Markus
Ströbele
a,
David
Enseling
b,
Thomas
Jüstel
b,
Michael
Westberg
c,
Peter R.
Ogilby
c and
H.-Jürgen
Meyer
*a
aAbteilung für Festkörperchemie und Theoretische Anorganische Chemie, Institut für Anorganische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany. E-mail: juergen.meyer@uni-tuebingen.de
bLabor für Angewandte Materialwissenschaften, Fachhochschule Münster, Stegerwaldstrasse 39, 48565 Steinfurt, Germany. E-mail: tj@fh-muenster.de
cDepartment of Chemistry, Aarhus University, Langelandsgade 140, 8000 Aarhus, Denmark. E-mail: progilby@chem.au.dk
First published on 5th September 2016
Abstract
Octahedral tungsten iodide clusters equipped with apical ligands (L) are synthesized to implement substantial photophysical properties. The [W6I8(CF3COO)6]2− cluster reported herein is the first example of a family of ligand substituted [W6I8L6]2− clusters. Such compounds are expected to exhibit a rich photochemistry in which the apical ligands play a crucial role. The versatile solid state and solution phase photophysical properties of (TBA)2[W6I8(CF3COO)6] described herein parallel characteristics obtained in some photophysically active organic compounds, including a broad absorption in the UV/VIS region. Upon irradiation of this compound, a broad red emission is observed in the VIS/NIR region resulting from excited triplet states, and singlet oxygen (a1Δg) is generated in the presence of O2.
Introduction
Optical materials are an essential part of our daily life; they offer attractive and energy-efficient applications in photocatalysis,1 sensing,2 lighting,3 and solar energy harvesting,4 for example. Thus, photoactive materials and studies of the properties of such materials with an eye to new developments are important.For the past decades, the electronic structure and photophysical properties of Mo6Cl12 based compounds have been studied.5 The structure of the [Mo6Cl14]2− ion is based on an octahedral molybdenum cluster core carrying eight inner (i = innen) face-capping and six outer (a = außen) apical chloride ligands making up a [Mo6Cl8iCl6a]2− ion which resembles the prototypical structure for many cluster compounds. Cluster compounds of this type are remarkably stable in solution and in the solid state. However, their apical ligands are always more weakly bound, allowing a broad substitutional chemistry for terminal ligands (L).
Considerable attention has focused on the structures and photophysical properties of octahedral metal cluster compounds [M6X8L6]n− with M = Mo, X = halide, and M = Re, X = S, Se or Te, containing inorganic or organic ligands (L).6
Octahedral rhenium chalcogenide clusters, developed almost simultaneously in 1999 by the groups of Kitamura,7 Batail8 and Nocera9 are showing versatile potential in optical applications.10 Likewise, a number of ligand substituted octahedral molybdenum clusters, such as (TBA)2[Mo6I8(C3F7COO)6]11 and (TBA)2[Mo6I8(CF3COO)6]12 (TBA = tetrabutyl ammonium), exhibit remarkable photophysical properties.13
The aforementioned metal chalcogenide and metal halide compounds have broad absorption bands in the UV-VIS region and emit in the red VIS/NIR region of the electromagnetic spectrum. It is clear that the photoexcitation involves an S0 to Sm transition, followed by intersystem crossing (ISC) into triplet states.14 Phosphorescence quenching obtained in the presence of molecular oxygen is, at least partially, explained by energy transfer with the formation of singlet oxygen, O2(a1Δg),15 as shown in Fig. 1.
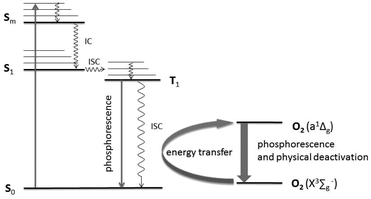 | ||
| Fig. 1 Electronic transitions and singlet oxygen generation (a1Δg) in [M6X8L6]2− type clusters following a modified Jablonski-Diagram.16 | ||
The photophysical and electrochemical properties of [M6X8L6]2− cluster compounds can be tuned by substitutions of inner ligands (X) as well as outer organic or inorganic ligands (L).12,14c,17 The perspectives of ligand exchange reactions in [M6X8L6] clusters and the influence of apical ligands with respect to the S0 → S1 excitation, described as ligand → metal charge transfer (LMCT)6b,18 and consequent photophysical effects are not fully explored. However, there is high potential and interest in [M6X8L6] cluster compounds regarding applications in the fields of oxygen sensing,5c catalysis,19 photocatalysis,20 photoreduction of CO2,21 luminescent nanoparticles,22 organic synthesis,23 and photodynamic therapy,24 for example.
Molybdenum clusters with the general formula [Mo6X8L6]2− have shown excellent luminescence properties. For the compound (TBA)2[Mo6I8(CF3COO)6], a luminescence quantum yield (ΦL) near 1 was reported in (oxygen-free) acetonitrile solution, and a quantum yield of 0.84 was reported for the sensitized production of singlet oxygen (ΦΔ) in the presence of oxygen.12,25
Corresponding tungsten iodide compounds [W6I8L6]2− remained absent until now. One reason is that there has been no appropriate synthesis available for the preparation of a binary tungsten iodide which could serve as a starting material (e.g. W6I12), although particularly strong luminescence properties of tungsten iodides, among [W6X6X′6]2− clusters with X, X′ = Cl, Br, I, have been observed.26 We here report the synthesis and photophysical characterization of a ligand substituted [W6I8L6]2− cluster compound.
Results and discussion
The development of a new synthetic route for tungsten iodides based on a halide exchange reaction of WCl6 with SiI4 enable us to generate tungsten iodide in large scale, based on W3I8·nI2 with n = 0, ½, 2.27 The octahedral cluster compound Cs2[W6I14] is prepared by thermal conversion of appropriate amounts of W3I12 and CsI in a sealed silica container near 550 °C.28An improved solubility in organic solvents is obtained by cation substitution reaction with (TBA)I, performed in a two phase solvent mixture (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water).29 (TBA)2[W6I14] is obtained as a bright yellow crystalline powder after phase separation and filtration from the organic phase. The crystal structure of (TBA)2[W6I14] was refined with the monoclinic space group P21/n from X-ray diffraction data with the FullProf program package.30 Average W–W distances of the octahedral cluster are 266.1 pm, W–I distances with inner iodide ligands average at 278.3 pm, and corresponding distances with apical iodide ligands are 285.5 pm.
water).29 (TBA)2[W6I14] is obtained as a bright yellow crystalline powder after phase separation and filtration from the organic phase. The crystal structure of (TBA)2[W6I14] was refined with the monoclinic space group P21/n from X-ray diffraction data with the FullProf program package.30 Average W–W distances of the octahedral cluster are 266.1 pm, W–I distances with inner iodide ligands average at 278.3 pm, and corresponding distances with apical iodide ligands are 285.5 pm.
(TBA)2[W6I14] as well as the starting material A2[W6I14] are key compounds for the development of ligand substituted [W6I8L6]2− cluster compounds with organic or inorganic ligands (L). Because it is unknown which ligand will give rise to the most promising photophysical properties with tungsten iodide clusters, we first attempted the preparation of a trifluoroacetate compound, as has been reported for the parent molybdenum compound.12 The exchange reaction of six outer iodide ligands in [W6I8iI6a]2− is performed with excess silver trifluoroacetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 molar ratio) in dichloromethane under stirring at room temperature for three days in the dark, following the scheme given in Fig. 2.
7 molar ratio) in dichloromethane under stirring at room temperature for three days in the dark, following the scheme given in Fig. 2.
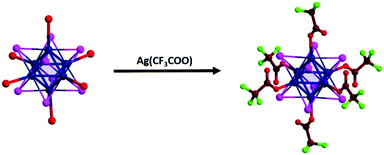 | ||
| Fig. 2 Exchange reaction of outer (a) iodide ligands in [W6I8iI6a]2− by trifluoroacetate with the formation of [W6I8(CF3COO)6]2−. | ||
(TBA)2[W6I8(CF3COO)6] is obtained as an orange colored crystalline solid with 79% yield. Good quality single crystals for X-ray structure analysis are grown from a saturated solution in ethanol by overlaying with pentane.
The identity of the cluster was confirmed from a (ESI) mass spectrum in methanol solution (Bruker Esquire 3000 plus), yielding mass peaks which are assigned to m/z = 3037.6 {(TBA)[W6I8(CF3COO)6]}−, and m/z = 2682.5 {[W6I8(CF3COO)5]}− fragments.
(TBA)2[W6I8(CF3COO)6] crystallizes with the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . [W6I8(CF3COO)6]2− ions are arranged in the bc-plane of the crystal, following the motive of a hexagonal closed packed layer, with a primitive stacking sequence along the b-axis direction. TBA ions can be approximated to occupy trigonal prismatic voids made up by the arrangement of clusters (Fig. 3).
. [W6I8(CF3COO)6]2− ions are arranged in the bc-plane of the crystal, following the motive of a hexagonal closed packed layer, with a primitive stacking sequence along the b-axis direction. TBA ions can be approximated to occupy trigonal prismatic voids made up by the arrangement of clusters (Fig. 3).
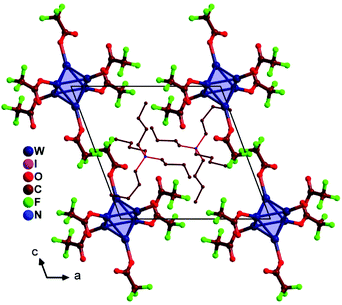 | ||
| Fig. 3 Crystal structure of (TBA)2[W6I8(CF3COO)6] projected on the ac-plane (iodide ligands are omitted). | ||
Tungsten atoms in the centrosymmetric [W6I8(CF3COO)6]2− cluster are arranged to form an octahedral arrangement with W–W distances ranging between 264.67(4) and 265.80(5) pm. Triangular cluster faces are capped by inner iodide ligands with W–I distances ranging from 278.48(6) to 282.24(6) pm. Three crystallographically distinct trifluoroacetate ligands appear in (TBA)2[W6I8(CF3COO)6] forming W–O–C angles of 135.1(6)°, 136.8(6)° and 134.8(6)° with tungsten atoms of the cluster. C–C distances within trifluoroacetate ligands range from 149(1) to 152(1) pm, consistent with a C–C single bond (148 pm), and C–C–F angles vary from 110.7(9)° to 115.2(12)°.
Crystalline powders of (TBA)2[W6I8(CF3COO)6] appear orange and show red-orange phosphorescence when exposed to UV radiation (Fig. 4). The phosphorescence can be considered an intrinsic property of the [W6I8(CF3COO)6]2− cluster anion, and the relative emission intensities of the crystalline powder continuously increases after milling with a highly reflective (inert) solid such as BaSO4. The quantum yield (ΦP) of (TBA)2[W6I8(CF3COO)6] is 4% as a pure microcrystalline powder, and 23% after milling with BaSO4, as determined in an integrating sphere in air.31 The increase of the luminescence intensity may be understood as a result of increased separation of clusters, which are considered as phosphorescence centers. This is in line with the observation of a considerably higher phosphorescence intensity once [W6I8(CF3COO)6]2− cluster anions are transferred into solution under inert atmosphere, as will be shown later.
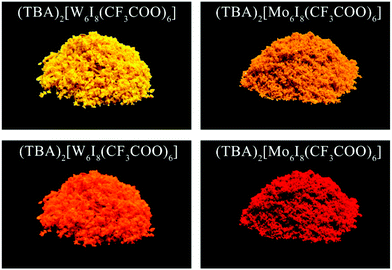 | ||
| Fig. 4 Crystalline powders of (TBA)2[W6I8(CF3COO)6] (left) and (TBA)2[Mo6I8(CF3COO)6] (right) under ambient light (top) and under UV radiation (366 nm, bottom). | ||
The emission spectrum of microcrystalline (TBA)2[W6I8(CF3COO)6 shows a broad emission band at 660 nm (1.88 eV) with a full width at half maximum (FWHM) of 0.43 eV (∼3500 cm−1) when excited with 400 nm (Fig. 5). The excitation spectrum of this emission band shows a maximum at 400 nm with full width at half maximum (FWHM) of 2.48 eV (∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1). A similar behavior is reported for (TBA)2[Mo6I8(CF3COO)6], which was prepared as a reference material.12
000 cm−1). A similar behavior is reported for (TBA)2[Mo6I8(CF3COO)6], which was prepared as a reference material.12
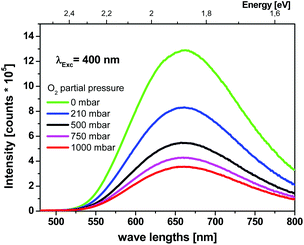 | ||
| Fig. 5 Emission spectra of microcrystalline (TBA)2[W6I8(CF3COO)6] powder under several oxygen partial pressures (λem,max = 660 nm). | ||
The large FWHM combined with a large Stokes shift of 1.12 eV (∼9000 cm−1) of (TBA)2[W6I8(CF3COO)6] can be related to a charge transfer transition.32 The phosphorescence of both compounds is quenched in the presence of oxygen. Emission spectra of (TBA)2[W6I8(CF3COO)6] recorded as a function of the oxygen partial pressure are shown in Fig. 5. The phosphorescence intensity as well as the decay time decrease with increasing oxygen partial pressure. The decay time of (TBA)2[W6I8(CF3COO)6] in pure nitrogen is 29.2 μs and in pure oxygen 9.9 μs (see ESI†). As the oxygen partial pressure is changed, the photoluminescence intensity as well as the decay time show reversibility within the limit of error of our measurements.
Because the quantum yield of emission is correlated with the decay time (Φ = τ/τr, where τ is the measured decay time and τr the decay time for the radiative transition),33 the relative decay time, τ, can be used to predict the relative change of the quantum yield.
Accordingly, the relative quantum yield of (TBA)2[W6I8(CF3COO)6] emission in pure oxygen atmosphere drops to 34% of the value obtained in pure nitrogen. A similar behavior is observed for (TBA)2[Mo6I8(CF3COO)6], where the relative quantum yield drops to 5%. The bimolecular oxygen-mediated quenching processes have been quantified via Stern–Volmer analysis (see Fig. 6). These results imply that this type of cluster compound could be considered as an oxygen sensor. Moreover, the oxygen mediated quenching of the phosphorescent triplet state could potentially lead to the formation of singlet oxygen, O2(a1Δg) (Fig. 1).
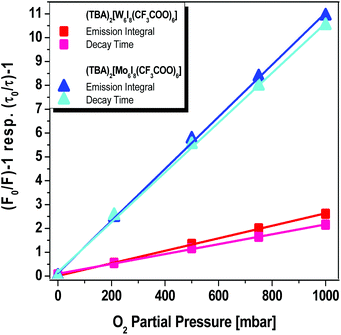 | ||
| Fig. 6 Stern–Volmer plots of the emission integrals as well as the decay times of microcrystalline (TBA)2[W6I8(CF3COO)6] and (TBA)2[Mo6I8(CF3COO)6] powder as a function of oxygen partial pressures. | ||
The photophysics of the compounds dissolved in acetonitrile was investigated (Table 2). Both clusters show a red-shifted emission spectrum, and the emission spectrum of (TBA)2[W6I8(CF3COO)6] is particularly broad and extends into the NIR (see ESI†). Phosphorescence lifetimes were determined at three different oxygen concentrations. From these lifetimes, it is possible to calculate the bimolecular rate constant for quenching of the triplet state by oxygen, kq. Even though the quenching process is not diffusion-limited, it can be calculated that more than 98% of the triplet states are quenched by oxygen under aerated conditions (fT, Table 2). This is clearly reflected in the very small phosphorescence quantum yields under these conditions.
| (TBA)2[W6I8(CF3COO)6] | |
|---|---|
| Empirical formula | C44H72F18I8N2O12W6 |
| Formula weight | 3281.33 |
| Crystal system |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a, b, c (pm) | 1308.62(4), 1321.46(4), 1335.44(4) |
| α, β, γ | 114.963(2)°, 102.910(3)°, 102.138(3)° |
| V (nm3) | 1.9157(1) |
| Z, Dx (g cm−3) | 1, 2.844 |
| μ (mm−1) | 12.285 |
| F(000) | 1476 |
| Crystal size (mm) | 0.2 × 0.1 × 0.1 |
| T max, Tmin | 0.4031, 0.1180 |
| Number of measured and independent reflections | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 913, 6690 913, 6690 |
| R int | 0.0188 |
| Θ max | 25.026° |
| Final R indices [I > 2σ(I)] | R 1 = 0.0348, wR2 = 0.0763 |
| Largest diff. peak and hole | 0.667 and −0.592 e Å−3 |
| (TBA)2[W6I8(CF3COO)6] | (TBA)2[Mo6I8(CF3COO)6] | ||
|---|---|---|---|
| λ em,max [nm] | 677 ± 2 | 675 ± 2 | |
| τ T [μs] | N2 | 36 ± 1 | 300 ± 10 |
| Air | 1.08 ± 0.02 | 1.84 ± 0.03 | |
| O2 | 0.23 ± 0.01 | 0.38 ± 0.01 | |
| k q [108 M−1s−1] | 3.7 ± 0.1 | 2.3 ± 0.1 | |
| f T | Air | 0.98 ± 0.01 | 0.99 ± 0.01 |
| Φ P | Air | 0.015 ± 0.003 | 0.004 ± 0.001 |
| Φ Δ | Air | 0.81 ± 0.06 | 0.84 ± 0.07 |
| O2 | 0.81 ± 0.06 | 0.85 ± 0.07 |
In order to check if this quenching process involves energy transfer to ground-state oxygen (X3Σg−), the quantum yields of O2(a1Δg) formation were determined via the characteristic 1275 nm phosphorescence signal from O2(a1Δg).16 Indeed, both clusters ([W6I8(CF3COO)6]2− and [Mo6I8(CF3COO)6]2−) are efficient O2(a1Δg) photosensitizers under aerated and oxygenated conditions (Table 2). However, for both clusters the observation that the singlet oxygen quantum yield is lower than the fraction of triplet states quenched by oxygen (ΦΔ < fT) implies that (1) the triplet quantum yield may be below unity and/or (2) some of the encounter complexes between oxygen and the hexanuclear clusters decay via a non-radiative mechanism instead of energy transfer to make singlet oxygen. Such mechanisms often dominate for photosensitizers with appreciable charge-transfer character.34 An interesting direction for future studies could therefore be to systematically develop [W6I8L6]2− cluster compounds and to investigate the effect of ligand-exchange on the singlet oxygen yields and charge-transfer character in the complexes.34c,d Furthermore, solvent-effects could potentially also tune the excited state properties of the clusters.34a,35
The functionalization of photophysically active clusters by means of deposition, incorporation, and encapsulation is a logical need for further applications.10
Experimental section
Materials and methods
Unless otherwise noted, all reactions were carried out in an argon atmosphere in dried and degassed solvents using Schlenk techniques. All solvents were purchased from Sigma-Aldrich, dried and degassed with an MBraun SPS-800 solvent purification system. Chemicals used were obtained from commercial suppliers and were used without further purification.X-ray crystallographic studies
Further details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (Fax: +49 7247-808-666; E-mail: crysdata@fiz-karlsruhe.de, http://www.fiz-karlsruhe.de/request for deposited data.html) on quoting the depository number CSD 429577 for Cs2[W6I14].
Crystallographic data (including structure factors) for (TBA)2[W6I14] and (TBA)2[W6I8(CF3COO)6] has been deposited with the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB12EZ, UK. Copies of the data can be obtained free of charge on quoting the depository number CCDC 1063061 for (TBA)2[W6I14] and CCDC 1479582 for (TBA)2[W6I8(CF3COO)6].
Solid state photoluminescence measurements
Emission spectra and decay curves were collected with a fluorescence spectrometer FLS920 (Edinburgh Instruments) equipped with a 450 W ozone-free xenon arc lamp (OSRAM) for the emission scan and a μF920H flash lamp for the decay curve. The sample chamber was installed with a mirror optic for powder samples. For detection, a R2658P single-photon counting photomultiplier tube (Hamamatsu) was used. All luminescence spectra were recorded with a spectral resolution of 1 nm, a dwell time of 0.4 s in 1 nm steps, and three repeats. The sample was in a gas flow sample holder under ambient pressure (about 1 bar) and was perfused with nitrogen, air, oxygen and oxygen–nitrogen mixtures 50/50 and 75/25.Solution phase spectroscopy
The compounds were dissolved in HPLC-grade acetonitrile (Sigma-Aldrich). Solutions were saturated with N2 or O2 by bubbling with the respective gas for at least 30 min. Phenalenone and 4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran (Sigma-Aldrich) was used as received. All experiments were performed in 1 cm quartz cuvettes.Equipment and methods used to determine singlet oxygen quantum yields, ΦΔ, have been described in detail elsewhere.39 In short, the chromophores were excited using fs-laser pulses centered at 383 nm, and photosensitization of O2(a1Δg) was follow via the characteristic phosphorescence signal at ∼1275 nm. This signal was isolated using a 1064 nm long-pass filter combined with a 1290/80 nm band-pass filter in front of a VIS/NIR-sensitive PMT. Phenalenone dissolved in acetonitrile was used as the reference sensitizer (ΦΔ = 0.99 ± 0.03).40
Phosphorescence lifetimes of the tungsten cluster were measured using the same instruments as described above. Excitation was done at either 383 or 419 nm, and the phosphorescence signals were isolated using a 650/40 nm band-pass filter. The bimolecular rate constant for quenching of the cluster triplet state by oxygen, kq, was calculated using a concentration of oxygen in air-saturated acetonitrile of 2.42 mM.41
Phosphorescence quantum yields of the cluster were measured using a home-built setup that allows for acquisition of emission spectra extending to 880 nm. This setup uses the same fs-laser as described above, but for these measurements the detection unit is an Andor Technology, iStar 320 T, ICCD camera connected to an Andor Technology, Shamrock 303i, spectrograph. The detection path is positioned at a 90 degrees angle relative to the excitation path. The detection unit has been intensity calibrated. 4-(Dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran in acetonitrile (ΦP = 0.44 ± 0.05)42 and absolute ethanol (ΦP = 0.435 ± 0.022)43 was used as reference standard.
Synthesis of Cs2[W6I14] and (TBA)2[W6I14]
| 2W3I12 + 2CsI → Cs2[W6I14] + 6I2 |
| Cs2[W6I14] + 2(TBA)I → (TBA)2[W6I14] + 2CsI |
769 mg (0.24 mmol) Cs2[W6I14] were added to a solution of 270 mg (0.73 mmol) (TBA)I in 100 mL CH2Cl2/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After stirring the mixture for 48 h the organic phase was separated and filtered. The solvent was removed and the precipitate was washed three times with distilled water. The orange solid was then again dissolved in DCM and again filtered. After removing the solvent (TBA)2[W6I14] was obtained as an orange solid in 79% yield.
1). After stirring the mixture for 48 h the organic phase was separated and filtered. The solvent was removed and the precipitate was washed three times with distilled water. The orange solid was then again dissolved in DCM and again filtered. After removing the solvent (TBA)2[W6I14] was obtained as an orange solid in 79% yield.
Synthesis of (TBA)2[W6I8(CF3COO)6]
| (TBA)2[W6I14] + 6Ag(CF3COO) → (TBA)2[W6I8(CF3COO)6] + 6AgI |
ESI-MS: m/z = 3037.6 {(TBA)[W6I8(CF3COO)6]}−; m/z = 2682.5 {[W6I8(CF3COO)5]}−
Conclusions
(TBA)2[W6I8(CF3COO)6] represents the first tungsten iodide cluster compound of a growing family of ligand substituted tungsten iodide clusters containing [W6I8L6]2− ions that are currently developed in Tübingen. The present compound exhibits remarkable stability in moist air. Recorded photophysical properties parallel those obtained for parent molybdenum clusters, represented by a red phosphorescence in solid state and in solution. The phosphorescence emission intensity is quenched with increasing concentration of molecular oxygen, with the formation of singlet oxygen in high yield.Acknowledgements
We are indebted to Mr Thorsten Hummel for the preparation of Cs2[W6I14] and to Dr Danuta Dutczak for the photographic art work.Notes and references
- J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang, Y. Libin, F. Wei, F. Honggang and S. Jiazhong, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773–1787 CrossRef.
- Y. Feng, J. Cheng, L. Zhou, X. Zhou and H. Xiang, Analyst, 2012, 137, 4885–4901 RSC.
- T. Jüstel, H. Nikol and C. Ronda, Angew. Chem., Int. Ed., 1998, 37, 3084–3103 CrossRef.
- M. Grätzel, Inorg. Chem., 2005, 44, 6841–6851 CrossRef PubMed.
- (a) J. C. Sheldon, J. Chem. Soc., 1960, 3106 RSC; (b) J. E. Fergusson, B. H. Robinson and C. J. Wilkins, J. Chem. Soc. A, 1967, 486–490 RSC; (c) D. J. Osborn and G. L. Baker, J. Sol–Gel Sci. Technol., 2005, 36, 5–10 CrossRef CAS.
- (a) W. A. Rabanal-León, J. A. Murillo-López, D. Páez-Hernández and R. Arratia-Pérez, J. Phys. Chem. A, 2014, 118, 11083–11089 CrossRef PubMed; (b) R. Ramirez-Tagle and R. Arratia-Pérez, Chem. Phys. Lett., 2008, 460, 438–441 CrossRef CAS.
- T. Yoshimura, S. Ishizaka, K. Umakoshi, Y. Sasaki, H.-B. Kim and N. Kitamura, Chem. Lett., 1999, 28, 697–698 CrossRef.
- C. Guilbaud, A. Deluzet, B. Domercq, P. Molinie, K. Boubekeur, P. Batail and C. Coulon, Chem. Commun., 1999, 1867–1868 RSC.
- T. G. Gray, C. M. Rudzinski, D. G. Nocera and R. H. Holm, Inorg. Chem., 1999, 38, 5932–5933 CrossRef CAS PubMed.
- S. Cordier, Y. Molard, K. A. Brylev, Y. Mironov, F. Grasset, B. Fabre and N. G. Naumov, J. Cluster Sci., 2015, 26, 53–81 CrossRef CAS.
- M. N. Sokolov, M. A. Mihailov, E. V. Peresypkina, K. A. Brylev, N. Kitamura and V. P. Fedin, Dalton Trans., 2011, 40, 6375–6377 RSC.
- K. Kaplan, P. Kubát, M. Dusek, K. Fejfarvová, V. Sícha, J. Mosinger and K. Lang, Eur. J. Inorg. Chem., 2012, 3107–3111 Search PubMed.
- (a) K. Harder and W. Preetz, Z. Anorg. Allg. Chem., 1992, 612, 97–100 CrossRef CAS; (b) W. Preetz, G. Peters and D. Bublitz, Chem. Rev., 1996, 96, 977–1026 CrossRef CAS PubMed.
- (a) T. Azumi and Y. Saito, J. Phys. Chem., 1988, 92, 1715–1721 CrossRef CAS; (b) A. Guirauden, I. Johannsen, P. Batail and C. Coulon, Inorg. Chem., 1993, 32, 2446–2452 CrossRef CAS; (c) A. W. Maverick, J. S. Najdzionek, D. MacKenzie, D. G. Nocera and H. B. Gray, J. Am. Chem. Soc., 1983, 105, 1878–1882 CrossRef CAS; (d) A. W. Maverick and H. B. Gray, J. Am. Chem. Soc., 1981, 103, 1298–1300 CrossRef CAS; (e) T. C. Zietlow, M. D. Hopkins and H. B. Gray, J. Solid State Chem., 1985, 57, 112–119 CrossRef CAS.
- J. A. Jackson, C. Turro, M. D. Newsham and D. G. Nocera, J. Phys. Chem., 1990, 94, 4500 CrossRef CAS.
- P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181–3209 RSC.
- T. C. Zietlow, M. D. Hopkins and H. B. Gray, J. Solid State Chem., 1985, 57, 112–119 CrossRef CAS.
- (a) L. M. Robinson, R. L. Bain, D. F. Shriver and D. E. Ellis, Inorg. Chem., 1995, 34, 5588–5596 CrossRef CAS; (b) L. F. Szczepura, J. A. Edwards and D. L. Cedeño, J. Cluster Sci., 2009, 20, 105–112 CrossRef CAS.
- S. Kamiguchi, S. Nagashima and T. Chihara, Metals, 2014, 4, 84 CrossRef.
- A. Barras, S. Cordier and R. Boukherroub, Appl. Catal., B, 2012, 123–124, 1–8 CrossRef CAS.
- P. Kumar, S. Kumar, S. Cordier, S. Paofai, R. Boukherroub and S. L. Jain, RSC Adv., 2014, 4, 10420–10423 RSC.
- T. Aubert, F. Cabello-Hurtado, M.-A. Esnault, C. Neaime, D. Lebret-Chauvel, S. Jeanne, P. Pellen, C. Roiland, L. Le Polles, N. Saito, K. Kimoto, H. Haneda, N. Ohashi, F. Grasset and S. Cordier, J. Phys. Chem. C, 2013, 117, 20154–20163 CAS.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351–371 CrossRef CAS.
- K. Kirakci, P. Kubát, M. Kučeráková, V. Šícha, H. Gbelcová, P. Lovecká, P. Grznárová, T. Ruml and K. Lang, Inorg. Chim. Acta, 2016, 441, 42–49 CrossRef CAS.
- K. Kirakci, P. Kubát, J. Langmaier, T. Polívka, M. Fuciman, K. Fejfarova and K. Lang, Dalton Trans., 2013, 42, 7224–7232 RSC.
- T. C. Zietlow, D. G. Nocera and H. B. Gray, Inorg. Chem., 1986, 25, 1351–1353 CrossRef CAS.
- M. Ströbele, C. Castro, R. F. Fink and H. J. Meyer, Angew. Chem., Int. Ed., 2016, 4894–4897 CrossRef.
- (a) K. Kirakci, S. Cordier and C. Perrin, Z. Anorg. Allg. Chem., 2005, 631, 411–416 CrossRef CAS; (b) T. Hummel, M. Ströbele, D. Schmid, D. Enseling, T. Jüstel and H.-J. Meyer, Eur. J. Inorg. Chem., 2016 Search PubMed , accepted.
- (a) M. K. Simsek, D. Bublitz and W. Preetz, Z. Anorg. Allg. Chem., 1997, 623, 1885–1891 CrossRef CAS; (b) M. K. Simsek and W. Preetz, Z. Anorg. Allg. Chem., 1997, 623, 515–523 CrossRef CAS.
- J. Rodríguez-Carvajal, Phys. B, 1993, 192, 55–69 CrossRef.
- Quantum yields
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) of (TBA)2[W6I8(CF3COO)6]: ϕp = 4% as pure microcrystalline powder; ϕp = 23% when dissolved in ethanol grindend with BaSO4.
of (TBA)2[W6I8(CF3COO)6]: ϕp = 4% as pure microcrystalline powder; ϕp = 23% when dissolved in ethanol grindend with BaSO4. - G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, Berlin, 1994 Search PubMed.
- G. Gauglitz and T. Vo-Dinh, Handbook of Spectroscopy, Wiley-VCH, Weinheim, 2003, vol. 1+2 Search PubMed.
- (a) M. Westberg, M. Bregnhøj, A. Blázquez-Castro, T. Breitenbach, M. Etzerodt and P. R. Ogilby, J. Photochem. Photobiol., A, 2016, 321, 297–308 CrossRef CAS; (b) C. Schweitzer and R. Schmidt, Chem. Rev., 2003, 103, 1685–1758 CrossRef CAS PubMed; (c) A. A. Abdel-Shafi, M. D. Ward and R. Schmidt, Dalton Trans., 2007, 2517–2527 RSC; (d) P. I. Djurovich, D. Murphy, M. E. Thompson, B. Hernandez, R. Gao, P. L. Hunt and M. Selke, Dalton Trans., 2007, 3763–3770 RSC; (e) P.-G. Jensen, J. Arnbjerg, L. P. Tolbod, R. Toftegaard and P. R. Ogilby, J. Phys. Chem. A, 2009, 113, 9965–9973 CrossRef CAS PubMed.
- S. Takizawa, T. Breitenbach, M. Westberg, L. Holmegaard, A. Gollmer, R. L. Jensen, S. Murata and P. R. Ogilby, Photochem. Photobiol. Sci., 2015, 14, 1831–1843 CAS.
- (a) G. M. Sheldrick, Acta Crystallogr., 1990, A46, 467 CrossRef CAS; (b) G. M. Sheldrick, SHELXTL, Bruker Analytical X-Ray Division, Madison, 2001 Search PubMed; (c) G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- P. Brückner, W. Preetz and M. Pünjer, Z. Anorg. Allg. Chem., 1997, 623, 8–17 CrossRef.
- T. Roisnel and J. Rodriguez-Carvajal, M. S. F. WinPLOTR: A Windows tool for powder diffraction patterns analysis, 378, 118–123. Recent developments of the program FULLPROF; Commission on powder diffraction (IUCr). Newsletter, 2001., WinPLOTR: A Windows tool for powder diffraction patterns analysis. Proceedings of the Seventh European Powder Diffraction Conference (EPDIC7), R. Delhez, E. J. Mittenmeijer, Eds.; Barcelona, Spain, May 20–23, 2000, vol. 118.
- (a) R. D. Scurlock, D. O. Martire, P. R. Ogilby, V. L. Taylor and R. L. Clough, Macromolecules, 1994, 27, 4787–4794 CrossRef CAS; (b) J. Arnbjerg, M. Johnsen, P. K. Frederiksen, S. E. Braslavsky and P. R. Ogilby, J. Phys. Chem. A, 2006, 110, 7375–7385 CrossRef CAS PubMed; (c) P. Salice, J. Arnbjerg, B. W. Pedersen, R. Toftegaard, L. Beverina, G. A. Pagani and P. R. Ogilby, J. Phys. Chem. A, 2010, 114, 2518–2525 CrossRef CAS PubMed.
- (a) R. Schmidt, C. Tanielian, R. Dunsbach and C. Wolff, J. Photochem. Photobiol., A, 1994, 79, 11–17 CrossRef CAS; (b) C. Martí, O. Jürgens, O. Cuenca, M. Casals and S. Nonell, J. Photochem. Photobiol., A, 1996, 97, 11–18 CrossRef.
- C. Franco and J. Olmsted, Talanta, 1990, 37, 905–909 CrossRef CAS PubMed.
- J. M. Drake, M. L. Lesiecki and D. M. Camaioni, Chem. Phys. Lett., 1985, 113, 530–534 CrossRef CAS.
- K. Rurack and M. Spieles, Anal. Chem., 2011, 83, 1232–1242 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1063061 and 1479582. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt02471h |
| This journal is © The Royal Society of Chemistry 2016 |
