Photochemical proton-coupled C–H activation: an example using aliphatic fluorination†
Abstract
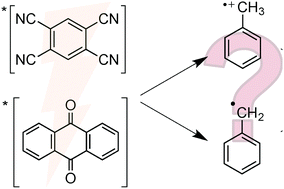
Selective functionalization of unactivated C–H bonds is an ongoing chemical challenge. C–H activation requires the transfer of H+ and e−, so called proton-coupled electron transfer (PCET) reactions. Recent efforts in photochemical PCET involving C–H bonds show great promise for the synthesis of new compounds. One such example is photochemical C–H fluorination reactions. In many cases, the discrete PCET mechanisms are yet to be defined in a systematic way. Here, we investigated electron transfer (ET) and PCET reactions of electronically excited 1,2,4,5-tetracyanobenzene and anthraquinone with the components of typical fluorination reactions. Analysis using kinetic and thermodynamic models, and steady-state and time-resolved fluorescence data, suggest that C–H activation proceeds efficiently where electronically excited sensitizers accept H˙.


 Please wait while we load your content...
Please wait while we load your content...