The impact of solvents on the singlet and triplet states of selected fluorine corroles – absorption, fluorescence, and optoacoustic studies†
Abstract
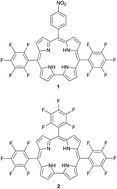
This paper examines the influence of aprotic solvents on the spectroscopic properties as well as the energy deactivation of two free-base corrole dyes substituted with C6F5 and/or 4-NO2C6H4 groups. Absorption, fluorescence and laser-induced optoacoustic spectroscopy have been used to follow the singlet and triplet states of fluorine corroles belonging to the A2B and A3 type in toluene (TL), chloroform (CL), dimethylformamide (DMF), dimethyl sulfoxide (DMSO) and also in solvent mixtures. Changes in the absorption and fluorescence spectra are influenced by the type of solvent mixture. The fluorescence behaviors of the two investigated corroles were extremely different – fluorescence of the nitro-corrole in TL is dramatically quenched in the presence of DMF. In contrast, fluorescence quenching of fluorine corroles in DMF–TL mixtures is substantially weakened. Absorption, fluorescence, triplet population as well as singlet oxygen generation parameters are evaluated. The spectral experimental data are supported by quantum chemical calculations – time-dependent density functional theory (TD-DFT) and cyclic voltammetry experiments. The presented results are discussed from a viewpoint of aggregation, tautomerization, and deprotonation effects occurring in the corroles.

 Please wait while we load your content...
Please wait while we load your content...