Predicting the composition and formation of solid products in lithium–sulfur batteries by using an experimental phase diagram†
Abstract
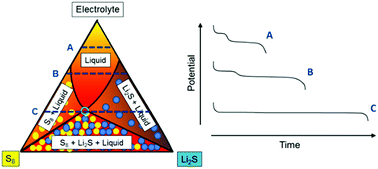
Lithium–sulfur batteries discharge via the transformation of solid sulfur to solid lithium sulfide via the formation of several polysulfide species that have only been observed in solution. Reported here is the first experimental phase diagram of a S8–Li2S-electrolyte system, which is shown to be a practical tool to determine the solution composition and formation of solid (S8 and Li2S) phases in lithium–sulfur batteries. The phase diagram is constructed by the combination of measurements of the total sulfur concentration [S]T and average oxidation state (Sm−) of polysulfide solutions prepared by reaction of S8 and Li2S. The phase diagram is used to predict the equilibrium discharge/charge profile of lithium–sulfur batteries as a function of the amount of electrolyte and the onset of precipitation and dissolution of solid products. High energy batteries should operate with a minimum amount of electrolyte, where both solid S8 and Li2S will be present during most of the charge and discharge of the cell, in which case we predict the observation of only one voltage plateau, instead of the two voltage plateaus commonly reported.

 Please wait while we load your content...
Please wait while we load your content...