Charge transport in nanoparticular thin films of zinc oxide and aluminum-doped zinc oxide†
Abstract
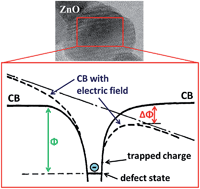
In this work, we report on the electrical characterization of nanoparticular thin films of zinc oxide (ZnO) and aluminum-doped ZnO (AZO). Temperature-dependent current–voltage measurements revealed that charge transport for both, ZnO and AZO, is well described by the Poole–Frenkel model and excellent agreement between the experimental data and the theoretical predictions is demonstrated. For the first time it is shown that the nature of the charge-transport is not affected by the doping of the nanoparticles and it is proposed that the Poole–Frenkel effect is an intrinsic and universally limiting mechanism for the charge transport in nanoparticular thin films with defect states within the bandgap.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry C Hot Papers

 Please wait while we load your content...
Please wait while we load your content...