Thermoresponsive PNIPAAm hydrogel scaffolds with encapsulated AuNPs show high analyte-trapping ability and tailored plasmonic properties for high sensing efficiency
A. C.
Manikas
a,
A.
Aliberti
a,
F.
Causa
*abc,
E.
Battista
a and
P. A.
Netti
abc
aCenter for Advanced Biomaterials for Healthcare@CRIB, Istituto Italiano di Tecnologia (IIT), Largo Barsanti e Matteucci 53, 80125 Naples, Italy. E-mail: causa@unina.it
bInterdisciplinary Research Centre on Biomaterials (CRIB), University “Federico II”, Piazzale Tecchio 80, 80125 Naples, Italy
cDipartimento di Ingegneria chimica, dei Materiali e della Produzione industriale, Piazzale Tecchio 80, 80125 Naples, Italy
First published on 22nd October 2014
Abstract
The fabrication of a scaffold able to control the positioning of AuNPs and to trap and concentrate target molecules inside them is a promising idea for a large variety of sensing applications. In this work, we designed and fabricated a scaffold of already-prepared 20 nm AuNPs encapsulated in a PNIPAAm hydrogel and utilizing surface enhanced Raman spectroscopy (SERS), we used it as a sensor with remarkably low limits of detection. In fact, as the target is trapped inside the hydrogel, the following takes place: (a) the concentration of the target increases dramatically and (b) the localization of the AuNPs and thus of the hotspots (areas with extremely high SERS enhancement factors) work synergistically, improving the sensing ability of the scaffold. The SERS enhancement ability of our scaffolds was checked with adenine, 2-naphthalenethiol and melamine molecules; the trapping efficiency was investigated for the melamine and a partition coefficient of k = 5 × 105 was found. Finally, by focusing on a single PNIPAAm hydrogel with encapsulated AuNPs, we managed to detect 10−6 M or rather 108 molecules of melamine trapped inside the scaffold.
Introduction
During the past decade, smart hydrogels have drawn enormous research interest in the biomedical and pharmaceutical field because they can adjust their volume and their properties in response to ambient stimuli.1,2 Among these, poly(N-isopropylacrylamide) (PNIPAAm) has been studied in detail with regard to its well-known phase behaviour in aqueous solutions, which has the sharpest transition in the class of thermosensitive alkylacrylamide polymers.3,4 Indeed, it undergoes a reversible phase transition, from a swollen to a shrunken state, with increasing temperature at about 32 °C in pure water. Below this temperature, called the lower critical solution temperature (LCST), PNIPAAm is hydrated and its chains are in an extended conformational state. Above the LCST, the hydrogel becomes dehydrated and collapses due to the breaking down of the hydrophilic–hydrophobic balance in its network structure. Dehydration takes place in the PNIPAAm, resulting in the aggregation of the PNIPAAm chain and leading to the shrinking of the hydrogel. These phase transitions induce dramatic modifications in the optical properties of the substrate.5Metallic nanoparticles (MNPs) possess distinct physical and chemical attributes that make them excellent scaffolds for the fabrication of novel chemical and biological sensors.6–8 MNPs can in fact be synthesized in a straightforward manner and made highly stable. Also, they possess unique optoelectronic properties and provide a high surface-to-volume ratio with excellent biocompatibility using appropriate ligands.9 Such properties can be readily tuned by varying the MNP size or shape and the surrounding chemical environment. For example, the binding event between the recognition element and the analyte can alter the physicochemical properties of transducer AuNPs, such as plasmon resonance absorption, conductivity, redox behaviour, etc., that in turn can generate a detectable response signal. MNPs can be multifunctionalized with a wide range of organic or biological ligands for the selective binding and detection of small molecules and biological targets.10,11 In the past decade of research, the advent of MNP as a sensory element has allowed for a broad spectrum of innovative approaches for the detection of metal ions, small molecules, proteins, nucleic acids, malignant cells, etc., in a rapid and efficient manner.12
Many hybrid materials are based on the polymer coating of preformed nanoparticles13–15 or the in situ synthesis of inorganic nanoparticles within a polymer matrix.16–18 So far, only a few studies19,20 have dealt with the loading of microgels with preformed nanoparticles. Lyon et al. loaded microgel particles with AuNPs in order to prepare hybrid materials for the photothermal patterning of colloidal crystals20 and for light-induced microlens formation.21 It was demonstrated that strong illumination of a small region of a concentrated sample leads to photothermal crystallization. However, neither the internal structure of the AuNP-loaded microgels nor any plasmon coupling effect during the volume phase transition of the AuNPs was investigated. Kumacheva et al. attached gold nanorods to copolymer microgels22 and showed that laser excitation of the longitudinal plasmon resonance of the gold nanorods can be used to induce a collapse of the microgel core. The optical properties of the hybrid particles during the volume phase transition were, however, not discussed. Using an approach similar to that of Kumacheva et al., Karg et al. attached polyelectrolyte-coated gold nanorods to the surface of oppositely charged microgels23,24 and the optical properties of the gold nanorods were studied as a function of microgel swelling. The microgel collapse led to a significant decrease in the surface area, thus reducing the distance between the attached gold nanorods. Plasmon coupling was observed below a certain nanorod spacing and the longitudinal plasmon resonance was found to be significantly red-shifted. Gawlitza et al. have recently presented plasmon coupling induced by an increase in temperature where AuNPs concentration and/or crosslinker density are directly related to the formation of dimers of AuNPs.25 To the best of our knowledge, SERS experiments exploiting the plasmon coupling induced by a temperature increase inside hybrid PNIPAAm–AuNPs templates, has not been reported till now. In our latest study, we have already presented a new method for easy and fast physisorption of AuNPs on the surface of PNIPAAm hydrogels and the formation of highly efficient SERS substrates.26 We now approach the challenging task of combining control over AuNP positioning and the capacity to trap27 and concentrate target molecules inside the scaffold. A new scaffold of 20 nm AuNPs encapsulated in a PNIPAAm hydrogel can be used as a sensor with low expected limits of detection. In fact, the increase of the trapped target and the localization of the hotspots (areas with extremely high SERS enhancement factors) inside the hydrogels work synergistically and improve the sensing ability of the scaffold.
The as-prepared scaffolds can be used for a large variety of applications because of the physical and chemical characteristics of both AuNPs and PNIPAAm hydrogels. We used them as sensors for the detection of different molecules by surface enhanced Raman spectroscopy because of their ability to localize the AuNPs within polymer matrices and tune their interparticle distance, thus inducing plasmon coupling.
Experimental
A. Materials
N-Isopropylacrylamide (97%) (NIPAM), N,N′-methylenebis(acrylamide) (BIS) (≥99.5%), potassium peroxodisulfate (KPS) (≥99%), adenine, melamine and 2-naphthalenethiol were purchased from Sigma-Aldrich (Munich, Germany). Gold nanoparticles (AuNPs) were from BBInternational. All the chemicals were used as received. A Millipore Milli-Q Plus 185 purification system was used for water purification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MW) against water for at least 1 week. This PNIPAAm microgel sample has a BIS feeding content of 2.5 wt%. Microgels with BIS contents of 4.5 wt% were synthesized using the same procedure, but different feeding amounts of BIS.
000 MW) against water for at least 1 week. This PNIPAAm microgel sample has a BIS feeding content of 2.5 wt%. Microgels with BIS contents of 4.5 wt% were synthesized using the same procedure, but different feeding amounts of BIS.
B. Methods
Using the Einstein–Batchelor relation:
| ηr = 1 + 2.5(k × c) + B(k × c)2 |
The data were fitted at a fixed temperature to obtain the intrinsic volume fraction:
| k = ζ/c |
Since the solution density is essentially equal to that of water, ρ = 1 g cm−3, the intrinsic volume fraction becomes:
| k = ζ/c = v/mp |
K values were calculated by viscosimetry and the volume of the particles v using dynamic light-scattering measurements.
Results and discussion
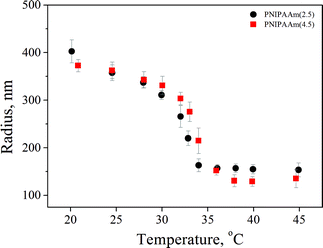
Two PNIPAAm hydrogels with different crosslinker concentrations of 2.5 and 4.5% were synthesized by precipitation polymerization. The samples are denoted PNIPAAm(x), where x describes the crosslinker concentration. The hydrodynamic radii were measured by DLS in a temperature range of 20 to 50 °C from the fully swollen to the fully collapsed state and the results are presented in Fig. 1. The particle mass necessary for the determination of the particle concentration was calculated using the Ubbelohde viscometer. The mesh size was also calculated as reported by Saunders32 and found to be ∼2 nm and ∼1.5 nm for PNIPAAm(2.5) and PNIPAAm(4.5), respectively. The results are presented in Table 1.| PNIPAAm(2.5) | PNIPAAm(4.5) | |
|---|---|---|
| Particle mass (g) | 2.58 × 10−12 | 8.45 × 10−12 |
| Particle concentration (Nparticles/mL) | 2.48 × 109 | 7.57 × 108 |
| Particle mesh size (nm) | 2 | 1.5 |
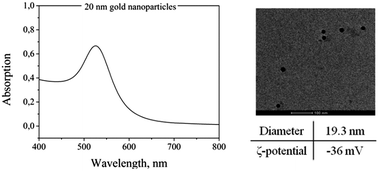
A deep characterization of the AuNPs is helpful before incorporating them in the PNIPAAm hydrogels. Specifically, TEM, UV-Vis spectroscopy and DLS measurements were performed on AuNP solutions. Fig. 2 shows a representative TEM image of the particles and a UV-Vis absorption spectrum with the typical absorption band at around 520 nm. The diameter of the particles measured from DLS is around 19.3 nm. The ζ-potential was also measured at 25 °C and found to be −36 mV.
Based on these results, we realized that it is impossible to physically trap the AuNPs inside the hydrogel because of the much larger dimensions of the nanoparticles compared to the hydrogel mesh size. So, an external stimulus was essential to force the AuNPs into the hydrogel and create the hybrid materials. Sonication was chosen as the external stimulus and the results are presented below.
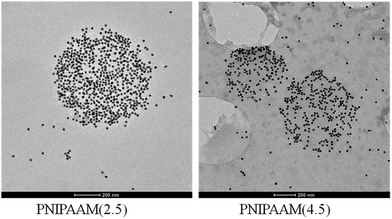
Transmission electron microscopy images of loaded PNIPAAm(2.5) and PNIPAAm(4.5) hydrogels at 10 min sonication time are presented in Fig. 3. We can observe that sonication allows the loading of the particles into the hydrogel. The PNIPAAm(2.5) hydrogel showed a greater loading efficiency because of its bigger mesh size and higher flexibility and was used for the following experiments.
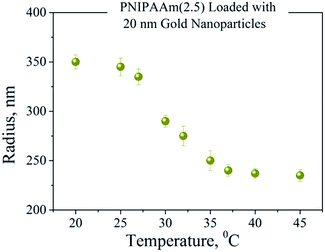
Thermoresponsive PNIPAAm–AuNPs composites were characterized by dynamic light scattering. Fig. 4 shows the variation of the hydrodynamic radius of PNIPAAm–AuNPs templates as the temperature rises from 15 to 40 °C. The critical temperature for our system was at around 33 °C, where the radius changed rapidly. Since the measured LCST is similar to that of the pure PNIPAAm hydrogel, the presence of Au nanoparticles does not significantly affect the swelling behavior of the PNIPAAm template. Specifically, the radius measured 340 nm for the completely swollen hydrogels at 15 °C and 230 nm for the collapsed ones at 40 °C, as clearly demonstrated in Fig. 4. The polydispersity of the synthesized templates measured 16% at the LCST, higher than that before the AuNPs adsorption, which was 11%.
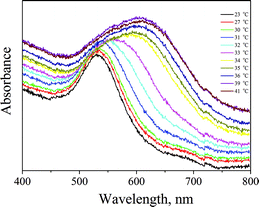
After verifying that the AuNPs were loaded inside the PNIPAAm hydrogel and after examining the size changes upon temperature variations, we investigated the plasmonic coupling of the surface plasmons. UV-Vis measurements were carried out on the aforementioned system at different temperatures and are presented in Fig. 5. The differences in plasmon resonance of AuNPs in the PNIPAAm hydrogel were fundamental for the fabrication of SERS-active substrates. It is clearly demonstrated in Fig. 5 that the absorption band of AuNPs at 525 nm changes as the temperature increases. More specifically, a continuous broadening of the band is visible until the LCST; above the LCST, changes are abrupt and the central absorption is red-shifted at around 620 nm. Such a red shift results from the coupling of surface plasmons between closely spaced particles; as the temperature rises, the distance between the AuNPs decreases. In aggregated colloids the particles are physically connected, but it is essential to note that direct contact is not always needed to observe collective plasmon modes. In fact, as long as the spacing between particles narrows compared to the wavelength of light, these collective plasmon modes can be observed. In this case, when the AuNPs–PNIPAAm template radius decreases, the interparticle spacing becomes narrow, resulting in the red shift of the absorption band.
The SERS efficiency of the fabricated hydrogel scaffolds was tested by different target molecules, such as adenine, melamine and 2-naphthalenethiol. 3 × 10−6 M adenine solution, 10−5 M melamine solution and 10−6 M 2-naphthalenethiol were added to PNIPAAm–AuNPs composites and allowed to diffuse in them. 100 μL (50 μL PNIPAAm–AuNPs and 50 μL of the target molecule) of this solution were placed under the microscope in a well plate. The spectra were collected using a 10×/0.25 objective at different temperatures. Representative spectra are presented in Fig. 6 demonstrating the intensity dependence upon temperature changes. As is clearly shown in Fig. 6, the intensity of the characteristic bands of each molecule at 20 °C is very low, but as the temperature increases, the intensity also increases significantly. Such a result was expected because when the size of the composites decreases, the AuNPs come closer to each other creating hotspots and resulting in high enhancement of Raman spectra. Differences in each molecule's main peak intensity vs. temperature are also presented in Fig. 6.
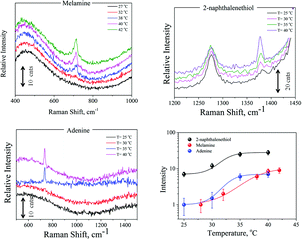 | ||
| Fig. 6 Representative SERS spectra of melamine, 2-naphthalenethiol, and adenine and also the intensity dependence on temperature changes. | ||
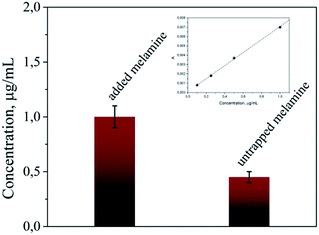
As we mentioned above, this scaffold has the ability to trap and concentrate the target molecule. For the investigation of the concentration efficiency, we added 200 μL of 2 μg mL−1 melamine solution to 200 μL AuNPs–PNIPAAm hydrogels. 2 h after the melamine addition, the solution was centrifuged and the concentration of melamine in the supernatant solution was measured by HPLC. As presented in Fig. 7, this concentration was 0.45 μg mL−1, meaning that a significant amount of the melamine got trapped inside the scaffold. Based on the amount of the target inside a single hydrogel and its volume, a concentration of the target of 1 M was calculated on average in each single hydrogel. These values show that there is a relevant concentration effect of the target inside the hydrogel; more specifically, a partition coefficient of k = 5 × 105 was found as a ratio of the inner to the outer melamine concentration. On an average, of 4.8 × 1014 molecules added to the solution, 3 × 1014 were trapped in the scaffold, while 1.8 × 1014 remained in the solution. This concentration effect is due to the high affinity of AuNPs with the amines of the melamine molecules33,34 that overcomes the negative entropic contribution of the concentrating phenomenon.
Taking into account the features of the fabricated scaffolds, we took one more step towards the improvement of their sensing efficiency. For the previously described SERS experiments we used 50 μL PNIPAAm–AuNPs. Such a volume contains around 106 PNIPAAm–AuNPs beads, but only a few of them interacted with the focusing field of the laser and gave us the information of the SERS spectra. Therefore, we isolated and focused on a single AuNP-loaded PNIPAAm hydrogel assuming to find similar spectra in a much lower amount of the target molecule. To test our assumptions, we placed the melamine solution with the AuNPs-loaded PNIPAAm hydrogels in Vitrotubes® rectangular capillaries and under a microscope. After 1 h of adsorption of the hydrogels on the bottom surface of the capillary, we focused the laser line with a 50×/0.50 LWD objective on a single hydrogel. The recorded spectra and the image are presented in Fig. 8; as expected, the intensity of the spectra was much higher. Specifically, as is clearly shown from the scale bars in Fig. 6 and 8, the increment of the melamine's Raman intensity, because of the better focusing, is around 4 times. In this case, the sensitivity of our system significantly increased and the LOD of the method is now less than 1 μM, much lower than the current melamine food safety requirements. Additionally, other melamine analytical methods35,36 dealing with a scaffold showed LODs comparable to or even higher than that presented in this approach. Furthermore, in our case the easier sample handling could lead to direct and straightforward use in clinical or food-testing applications. On the other hand, by focusing on a single hydrogel, the detectable quantity of melamine is extremely low and thus the experimentally required amount of melamine was also much lower. Spectral contribution of melamine molecules outside the hydrogels was not possible because of the extremely low melamine concentration for the acquisition of conventional Raman spectra. The acquired spectra come exclusively from melamine molecules close to SERS-active substrates, present only inside the loaded PNIPAAm–AuNPs hydrogels. The proposed experimental methodology offers the possibility of ultrasensitive detection due to the entrapment and the concentration of the target molecules inside the PNIPAAm–AuNP hydrogels and the activation of the hot-spots when increasing the temperature. This procedure can be generalized for a large number of molecules that can be trapped in our scaffold by physical adsorption, by diffusion, or even by chemical binding using gold nanoparticles conjugated with specific antibodies for more specific sensing applications. With respect to the specificity, in the case of analogues of the target molecules or interferents, Raman spectroscopy possesses the capability to discriminate even very similar molecules that might be trapped in the hydrogel template.
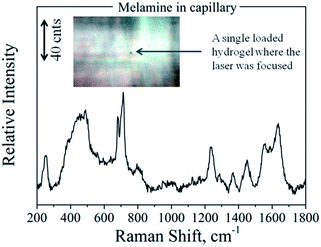 | ||
| Fig. 8 SERS spectra of melamine inside a glass capillary focused on a single loaded PNIPAAm hydrogel, as presented in the inset photo. | ||
Conclusions
In conclusion, in this work we designed and fabricated new scaffolds of 20 nm AuNPs encapsulated in a PNIPAAm hydrogel that can be used for a large variety of sensing applications. In our case, such scaffolds were used as sensors for the detection of different molecules by surface-enhanced Raman spectroscopy because of their ability to localize the AuNPs within polymer matrices and tune their interparticle distance, thus inducing plasmon coupling. In fact, as the target is trapped inside the hydrogel, the following takes place: (a) its local concentration increases dramatically and (b) the presence of the AuNPs and their localization inside the thermoresponsive scaffold generates hotspots (areas with extremely high enhancement factors) upon temperature variations. These two factors work synergistically, significantly improving the sensing ability of the scaffold. Furthermore, by focusing on a single loaded hydrogel, we further improved the sensitivity of the system by around 4 times because of the better focusing and reduced sampling volume. This approach can be adopted for every molecule with specificity to AuNPs or to PNIPAAm hydrogels in which it can be trapped, with extremely low limits of detection. Chemical modifications of the hydrogels or of the gold nanoparticles could also increase significantly the number of different target molecules that can be trapped inside them, making detection possible at extremely low concentration levels.Notes and references
- D. Q. Wu, Y. X. Sun, X. D. Xu, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, Biomacromolecules, 2008, 9, 1155 CrossRef CAS PubMed
.
- J. Zhang and N. A. Peppas, Macromolecules, 2000, 33, 102 CrossRef CAS
.
- T. Wu, Q. Q. Zhang, J. M. Hu, G. Y. Zhang and S. Y. Liu, J. Mater. Chem., 2012, 22, 5155 RSC
.
- J. H. Kim and T. R. Lee, Langmuir, 2007, 23, 6504 CrossRef CAS PubMed
.
- R. A. Alvarez-Puebla, R. Contreras-Caceres, I. Pastoriza-Santos, J. Perez-Suste and L. M. Liz-Marzan, Angew. Chem., Int. Ed., 2009, 48, 138 CrossRef CAS PubMed
.
- E. Boisselier and D. Asturc, Chem. Soc. Rev., 2009, 38, 1759 RSC
.
- H. Haick, J. Phys. D: Appl. Phys., 2007, 40, 7173 CrossRef CAS
.
- M. Zayats, R. Baron, I. Popov and I. Willner, Nano Lett., 2005, 5, 21 CrossRef CAS PubMed
.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed
.
- S. H. Radwan and H. M. E. Azzazy, Expert Rev. Mol. Diagn., 2009, 9, 511 CrossRef CAS PubMed
.
- A. C. Manikas, F. Causa, R. Della Moglie and P. A. Netti, ACS Appl. Mater. Interfaces, 2013, 5, 7915–7922 CAS
.
- R. Wilson, Chem. Soc. Rev., 2008, 37, 2028 RSC
.
- R. Contreras-Caceres, A. Sanchez-Inglesias, M. Karg, J. Perez-Juste, J. Pacifico, T. Hellweg, A. Fernandez-Barbero and L. M. Liz-Marzan, Adv. Mater., 2009, 20, 1666 CrossRef
.
- M. Karg, I. Pastoriza-Santos, L. M. Liz-Marzan and T. Hellweg, ChemPhysChem, 2006, 7, 2298 CrossRef CAS PubMed
.
- M. Karg, S. Jaber, T. Hellweg and P. Mulvaney, Langmuir, 2011, 27, 820 CrossRef CAS PubMed
.
- A. Pich, A. Karak, Y. Lu, A. K. Ghosh and H. J. P. Adler, Macromol. Rapid Commun., 2006, 27, 344 CrossRef CAS
.
- Y. Lu, S. Proch, M. Schrinner, M. Drechsler, R. Kempe and M. Ballauff, J. Mater. Chem., 2009, 19, 3955 RSC
.
- H. Lange, B. H. Juarez, A. Carl, M. Richter, N. G. Bastus, H. Weller, C. Thomsen, R. Von Klitzing and A. Knorr, Langmuir, 2012, 24, 8862 CrossRef PubMed
.
- J. H. Lee, A. M. A. Mahmoud, V. Sitterle, J. Sitterle and J. Carson Meredith, J. Am. Chem. Soc., 2009, 131, 5048 CrossRef CAS PubMed
.
- C. Jones and L. Lyon, J. Am. Chem. Soc., 2003, 125, 460 CrossRef CAS PubMed
.
- C. Jones, M. Serpe, L. Schroeder and L. Lyon, J. Am. Chem. Soc., 2003, 125, 5292 CrossRef CAS PubMed
.
- M. Das, N. Sanson, D. Fava and E. Kumacheva, Langmuir, 2007, 23, 196 CrossRef CAS PubMed
.
- M. Karg, I. Pastoriza-Santos, J. Perez-Juste, T. Hellweg and L. M. Liz-Marzan, Small, 2007, 3, 1222 CrossRef CAS PubMed
.
- M. Karg, Y. Lu, E. Carb-Argibay, I. Pastoriza-Santos, J. Perez-Juste, L. M. Liz-Marzan and T. Hellweg, Langmuir, 2009, 25, 3163 CrossRef CAS PubMed
.
- K. Gawlitza, S. T. Turner, F. Polzer, S. Wellert, M. Karg, P. Mulvaney and R. Von Klitzing, Phys. Chem. Chem. Phys., 2013, 15, 15623 RSC
.
- A. C. Manikas, G. Romeo, A. Papa and P. A. Netti, Langmuir, 2014, 30, 3869 CrossRef CAS PubMed
.
- R. Riahi, K. E. Mach, R. Mohan, J. C. Liao and P. K. Wong, Anal. Chem., 2011, 83, 6349 CrossRef CAS PubMed
.
- M. J. Sepre, C. D. Jones and L. A. Lyon, Langmuir, 2003, 19, 8759 CrossRef
.
- X. J. Gong, C. Wu and T. Ngai, Colloid Polym. Sci., 2010, 288, 1167 CAS
.
- C. Jones and L. Lyon, Macromolecules, 2000, 33, 8301 CrossRef CAS
.
- G. Romeo, L. Imperiali, J. Kim, A. Fernandez-Nieves and D. A. Weitz, J. Chem. Phys., 2012, 136, 124905 CrossRef PubMed
.
- B. R. Saunders, Langmuir, 2004, 20, 3925 CrossRef CAS
.
- A. Kumar, S. Mandal, P. R. Selvakannan, R. Pasricha, A. B. Mandale and M. Sastry, Langmuir, 2003, 19, 6277 CrossRef CAS
.
- D. V. Leff, L. Brandt and J. R. Heath, Langmuir, 1996, 12, 4723 CrossRef CAS
.
- J. M. Li, W. F. Ma, C. Wei, L. Y. You, J. Guo, J. Hu and C. C. Wang, Langmuir, 2011, 27, 14539 CrossRef CAS PubMed
.
- J. F. Betz, Y. Cheng and G. W. Rubloff, Analyst, 2012, 137, 826 RSC
.
| This journal is © The Royal Society of Chemistry 2015 |