High electrical conductivity of double-walled carbon nanotube fibers by hydrogen peroxide treatments†
A.
Morelos-Gómez‡
*ab,
M.
Fujishige
a,
S.
Magdalena Vega-Díaz
c,
I.
Ito‡
ab,
T.
Fukuyo
d,
R.
Cruz-Silva‡
bc,
F.
Tristán-López
c,
K.
Fujisawa
e,
T.
Fujimori
c,
R.
Futamura
c,
K.
Kaneko
c,
K.
Takeuchi
*ab,
T.
Hayashi
e,
Y. A.
Kim
f,
M.
Terrones
cg,
M.
Endo
*ab and
M. S.
Dresselhaus
h
aInstitute of Carbon Science and Technology, Shinshu University, 4-17-1 Wakasato, Nagano-city 380-8553, Japan. E-mail: endo@endomoribu.shinshu-u.ac.jp; takeuchi@endomoribu.shinshu-u.ac.jp; amorelos@shinshu-u.ac.jp
bGlobal Aqua Innovation Center, Shinshu University, 4-17-1 Wakasato, Nagano 380-8553, Japan
cCONACYT Research Fellow Technological Institute of Celaya, Department of Chemical Engineering, Avenida Tecnologico esq. A. Garcia Cubas S/N, C.P. 38010, Celaya, Guanajuato, Mexico
dMEFS Co., Ltd, Nagano-city 380-0921, Japan
eFaculty of Engineering, Shinshu University, 4-17-1 Wakasato, Nagano-city 380-8553, Japan
fSchool of Polymer Science and Engineering, Chonnam National University, 77 Yongbong-ro, Buk-gu, Gwangju, 500-757, Korea
gDepartment of Physics, Department of Chemistry, Department of Material Science and Engineering, Center for 2-Dimensional and Layered Materials, The Pennsylvania State University, University Park, Pennsylvania 16802, USA
hDepartment of Electrical Engineering and Computer Science, Department of Physics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139-4307, USA
First published on 29th October 2015
Abstract
Double-walled carbon nanotube (DWNT) fibers are of great interest due to their electrical properties and light weight, making them attractive for industrial applications including their potential use in power transmission lines. We present here a detailed study of the mechanism by which hydrogen peroxide (H2O2) treatment improves the electrical transport of DWNT fibers. These fibers were immersed and sonicated in H2O2 for several hours. Experimental results suggest that residual H2O2 could be intercalated within intertube channels inside the bundles of DWNTs, and the oxidation treatment could also result in the removal of small diameter carbon nanotubes (CNTs). In addition, an increase in the fiber density resulted in a decrease of the electrical resistivity. The H2O2 treatment of the DWNT fibers resulted in a metallic-like temperature dependent resistivity behavior with a transition to a semiconducting-like behavior below 30 K. We compared the effects of H2O2 with other well-known solvents and additives commonly used to reduce the carbon nanotube fiber electrical resistivity and found that the electrical conductivity values observed in our study are as good as those obtained with thionyl chloride and iodine additives. The H2O2 method was also used to treat other forms of carbon, where only the multi-walled carbon nanotubes doped with nitrogen exhibited a decrease in electrical resistivity. The fabrication method presented here is simple, efficient and low cost, thus making it an ideal process to be applied in the fabrication of electrically conducting carbon nanotube fibers.
Introduction
Since the development of the well-established production process for nanoscale carbon fibers based on the catalytic chemical vapor deposition (CCVD) method,1,2 and the subsequent recognition that these fibers are members of the fullerene family3,4 now called carbon nanotubes (CNTs), the electrical and mechanical properties of single-, double- and multi-walled carbon nanotubes (SWNTs, DWNTs and MWNTs) have been widely and actively studied.5–7 One of the outstanding properties of these materials is their high electrical conductivity. For example, SWNT-based ropes may exhibit electrical resistivity as low as 10−4 Ω cm.8–10 In particular, pristine DWNTs could exhibit electrical resistivity values ranging between 10−2 Ω cm and 10−4 Ω cm,11,12 with lower electrical resistivities than SWNTs.13 Unfortunately, many synthetic methods yield a mixture of semiconducting and metallic CNTs, and in addition, catalyst nanoparticles and amorphous carbon could be present within the samples. The different types of nanotubes and impurities from the synthesis will affect the electrical properties, resulting in increased resistivity values. Therefore, further research needs to be conducted in order to address these critical issues.Doping and intercalation processes have been widely used to decrease the electrical resistivity of CNTs, in a similar way as is done in graphite intercalation compounds.14 One approach consists of exohedral doping with potassium, bromine or iodine. CNTs doped with potassium or bromine could exhibit resistivity values ranging between 10−3 and 10−4 Ω cm.15,16 In particular, DWNT ropes doped with iodine could yield low electrical resistivities in the order of 10−5 Ω cm.11 Other chemical treatments with molecules, such as HNO3, H2SO3, H2SO4, NH4S2O8, SOCl2, yielded electrical resistivity values ranging between 10−3 and 10−5 Ω cm.13,17–22 In-plane doping, by the substitution of carbon atoms with nitrogen atoms could also decrease the electrical resistivity of CNTs to values of ca. 2.4 × 10−3 Ω cm.23 Unfortunately, the majority of the molecules used in these procedures are highly toxic, and could be hazardous and risky for the development of large-scale applications. In addition, various intercalation methods require the use of controlled atmospheres because of their air sensitivity and instability at elevated temperatures.
H2O2 is well known to react mildly with graphitic carbon and to purify carbon nanotube samples,11,12 and could decrease the electrical resistivity of DWNTs to 10−3 Ω cm.12 Wei et al. attributed the decrease of the electrical resistivity due to the surface purification of their samples; however, they did not consider other possible mechanisms.12 Zhao et al. also used H2O2 for the purification of their DWNT fiber.11 However, they thermally oxidize their sample before the H2O2 treatment. Afterwards, they continue to treat their sample with HCl. Unfortunately, they did not study the intermediate steps (thermal oxidation, hydrogen peroxide treatment and HCl treatment) to monitor how the electrical resistivity changes during each step and how each intermediate step contributes to understanding the role of the overall H2O2 treatment.
H2O2 treatments have been reported to remove small diameter semiconducting SWNTs by selective oxidation, when the suspensions were heated between 50 °C and 90 °C, and in a particular case aided by laser irradiation.24,25 Here, we report a detailed study aimed at elucidating the mechanism whereby the electrical resistivity of DWNT ropes sonicated with H2O2 is reduced. Our results indicate that the decrease in resistivity is caused by the removal of small diameter semiconducting CNTs and by an increase in the fiber density that could further enhance the electrical transport within DWNTs. We found that the H2O2 treatment could reduce the electrical resistivity from the pristine value of 4.19 × 10−3 Ω cm to 2.77 × 10−4 Ω cm. This method is low cost, simple, and effective to reach low electrical resistivity values that are commercially attractive, and more importantly, could be easily scaled up. In addition, this method is safer than others which rely on more aggressive solvents and additives, such as thionyl chloride, iodine, nitric acid, sulfuric acid, among others.
Experimental
Double walled nanotubes (DWNTs) were synthesized by chemical vapor deposition (CVD) with a floating catalyst process.1,2 The carbon feedstock contained decahydronaphthalene, thiophene and ferrocene, with a ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
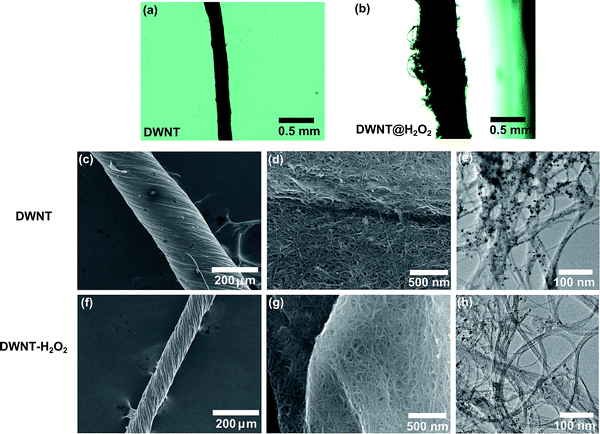
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. The CVD reaction was carried out in the presence of hydrogen at a temperature of 1300 °C. The obtained material (Fig. 1) was in the form of a DWNT net form.
1.5. The CVD reaction was carried out in the presence of hydrogen at a temperature of 1300 °C. The obtained material (Fig. 1) was in the form of a DWNT net form.
The net was twisted by hand and soaked in acetone (Fig. 1c–e), to allow certain shrinkage during the acetone evaporation; this sample is labeled as a DWNT. The DWNT was then immersed in hydrogen peroxide (34.5%) and sonicated in an ultrasonic bath for a time between 20 and 30 hours. After sonication, the sample was dried at 90 °C and soaked in acetone for further twisting; this sample is labeled as DWNT–H2O2 (Fig. 1f–h). For other treatments, the fibers were also twisted in acetone after the treatment. For chemical doping, the DWNT fiber was sonicated in SOCl2 (95%) and in NH4OH (28%) for 3 hours, and then dried; shorter times were used due to the complete decomposition of the fiber morphology after prolonged sonication. The silver nanoparticles were synthesized following the method reported by Coskun et al.26 Here 10 ml of 0.45 M ethylene glycol solution of poly(vinylpyrrolidone) (MW = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), was mixed and then 7 mg of NaCl was added at room temperature. Subsequently, the solution was heated to 170 °C and 0.21 M of AgNO3 in ethylene glycol was added manually dropwise into the first solution accompanied by magnetic stirring at ca. 1000 rpm. The solution was left at 170 °C for 30 minutes and then left to cool to room temperature. Finally, the solution was centrifuged at 4000 rpm and the supernatant was collected. Later the fibers were soaked in the supernatant solution containing the silver particles, then dried and heat treated at 300 °C in air in order to remove impurities. Iodine doping was carried out following the procedure published by Zhao et al.11 DWNT fibers were placed in the iodine vapor at 473 K for 3 h. The DWNT fibers were placed in a stainless steel container with 0.5 g of iodine powder. Prior to heating, the container was evacuated to approximately 5 kPa: iodine vaporizes at 0.14 MPa at the temperature 473 K. For the heat treatment of DWNT–H2O2 in air, the fiber was placed in the furnace at room temperature, heated to the desired temperature and withdrawn from the furnace after 15 minutes.
000), was mixed and then 7 mg of NaCl was added at room temperature. Subsequently, the solution was heated to 170 °C and 0.21 M of AgNO3 in ethylene glycol was added manually dropwise into the first solution accompanied by magnetic stirring at ca. 1000 rpm. The solution was left at 170 °C for 30 minutes and then left to cool to room temperature. Finally, the solution was centrifuged at 4000 rpm and the supernatant was collected. Later the fibers were soaked in the supernatant solution containing the silver particles, then dried and heat treated at 300 °C in air in order to remove impurities. Iodine doping was carried out following the procedure published by Zhao et al.11 DWNT fibers were placed in the iodine vapor at 473 K for 3 h. The DWNT fibers were placed in a stainless steel container with 0.5 g of iodine powder. Prior to heating, the container was evacuated to approximately 5 kPa: iodine vaporizes at 0.14 MPa at the temperature 473 K. For the heat treatment of DWNT–H2O2 in air, the fiber was placed in the furnace at room temperature, heated to the desired temperature and withdrawn from the furnace after 15 minutes.
The SWNTs used in this study are purified showing metallic (98%) and semiconductor (98%) content as purchased from Nano Integris in the form of buckypaper, the percentages reflect the metallic to semiconductor ratio or vice versa. The highly oriented pyrolytic graphite (HOPG) from SPI (Grade 1), and the graphite film is from the Panasonic Corporation. Multiwalled carbon nanotubes were synthesized by CVD with a mixture toluene/ethanol/ferrocene, and are labeled as MWNT–COx.27 MWNTs doped with nitrogen were synthesized by CVD with a mixture of benzylamine/ferrocene, and labeled as MWNT–CNx.28 The polyacrylonitrile (PAN) fibers T-300, were fabricated by Toray Industries, Inc. These carbon materials were held only to the H2O2 treatment, as described before.
The DWNT and DWNT–H2O2 samples were characterized by scanning electron microscopy (SEM) (HR-SEM, JSM-7000F/1V, JEOL), Raman spectroscopy (Renishaw in Via Raman microscope, 532 nm and 785 nm and T64000, Horiba Jobin Yvon 488 nm, 633 nm, 1064 nm). The fibers were dispersed ultrasonically in isopropanol in order to carry out transmission electron microscopy (TEM) studies (Cs-corrector (2) equipped with HR-TEM, JEM2100, JEOL, Japan). Surface chemical analysis was performed by X-ray photoelectron spectroscopy (XPS) using an Axis-Ultra, Kratos, UK apparatus. X-ray diffraction data was measured using a synchrotron radiation source at beam line BL04B2 of SPring-8 in Japan, where the X-ray wavelength is 0.0328 nm. Tensile tests were carried out with a Micro Strain Tester (Micro Autograph MST-I, Shimadzu). Resistivity and magnetoresistance measurements of the different fiber samples were carried out using a Quantum Design physical properties measurement system (PPMS), where a four-probe configuration was used by attaching the electrodes with silver paint with an inner electrode distance of approximately 1.5 mm. The applied current was fixed at 100 μA and the voltage was monitored. Prior to the measurements, the fibers were heated up to 100 °C for one hour within the equipment. The resistivity measurements were carried out between 380 K and 2 K, and the magnetoresistance measurements were made between −6 and 6 T at 2 K.
Results & discussion
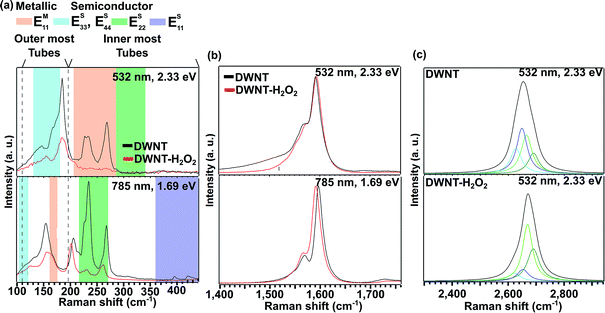
Scanning electron microscopy (SEM) images depict DWNT fibers before and after the H2O2 treatment (see Fig. 1). The pristine DWNT fiber was soaked in acetone and twisted, giving an average fiber diameter of ca. 217 μm (Fig. 1a–d). The resulting DWNT fiber was then sonicated in H2O2 and dried in air, and during the H2O2 treatment the fiber exhibits a volume expansion due to a partial de-bundling of the DWNTs (diameter ca. 500 μm, Fig. 1b), due to the H2O2 wetting; a volume expansion is also observed when soaking DWNTs in sulfuric acid.11 When drying, the fibers became “flat” on the drying substrate due to the removal of the solvent and to the surface tension during evaporation. Therefore, the fiber was soaked in acetone again and twisted after the H2O2 treatment, in order to obtain a fiber that could be handled for further characterization (average fiber diameter of ca. 95 μm, Fig. 1f and g). The densities of the fibers, before and after the H2O2 treatment with further twisting, increased from 0.21 g cm−3 to 1.29 g cm−3. This densification could partially aid in the decrease in electrical resistivity, providing an increase in ohmic contact among DWNTs throughout the entire fiber. From the SEM studies, a reduction of the metal nanoparticle content within the fibers was observed for the DWNT–H2O2 in Fig. 1h relative to Fig. 1e. Transmission electron microscope (TEM) images reveal that pristine DWNTs initially contained some amorphous carbon as well as iron nanoparticles encapsulated within carbon shells (see Fig. SI1†). After the H2O2 treatment some of the DWNTs break and many of these iron nanoparticles were removed, leaving behind hollow carbon shells and amorphous carbon (see Fig. SI1e and f†).11,12 The area of the carbon materials and iron was measured from the TEM images and the iron content area was calculated. DWNT and DWNT–H2O2 fibers exhibit a content of 1.28% and 0.71% iron based on the area of the iron nanoparticles from the TEM images, respectively (see Fig. 1e and h). Hydrogen peroxide cracks the amorphous carbon enabling the catalyst particles to be exposed and to react with H2O2 following the Fenton reaction.29,30 The encapsulation of the iron particles could decrease their reactivity, therefore leaving amorphous carbon and carbon shell material within the fiber. In addition, some DWNTs partially break as a result of these treatments.We recorded Raman spectra using different laser excitation energies (2.33 eV and 1.96 eV) on DWNT fibers before and after H2O2 treatment in order to monitor structural changes, and to observe different behaviors in the radial breathing modes. In Fig. 2a, the radial breathing mode (RBM) is depicted. Here two regions can be observed: the inner walls above 190 cm−1 (1.2 nm) and the outer walls below 190 cm−1.31–33 There are four possible configurations for semiconducting and metallic tubes within DWNTs (i.e., S@M, S@S, M@M and M@S). It is important to emphasize that an inner S tube that is in resonance with a certain Elaser will possess S or M outer tubes that in general are not in resonance with the same Elaser as the inner tube, and the same situation applies to inner M tubes.32 The diameters were calculated according to the equation ωRBM = 218.3/dt + 15.9, where ωRBM is in cm−1.32–34 The assignment of the outer and inner nanotubes was carried out by TEM observations, where the full width half maximum (FWHM) linewidths for the outer tubes were between 1.16 nm and 1.83 nm and for the inner tubes were between 0.52 nm to 1.32 nm (see Fig. SI2†). Fig. 2a highlights the transition energies at different laser energies according to the theoretical Kataura plot.35,36 The semiconducting nanotubes with diameters between 0.86 nm and 1 nm have Raman peak intensities in the range 220–270 cm−1 and are closer to their resonant energies when irradiated with the 1.69 eV laser, while the peak intensities for the outer tubes were observed mainly with 2.33 eV laser irradiation both for the pristine DWNTs and for the H2O2 treated DWNTs. With both laser energies it is evident that their intensities are greatly decreased when treated with H2O2. The variation in intensity could arise from the oxidation using H2O2 and from a decrease in the content of small diameter CNTs.27,28,37,38 Doping effects from residual H2O2 or other by-products from the chemical treatment are not considered because the Raman measurements were made after drying the samples.
The D-band region suggests disorder and symmetry-breaking within carbon materials. It is well known that H2O2 can act as a soft oxidizer for carbon materials. Therefore, after several hours of H2O2 treatment some structural disorder could arise within the sample due to breakage of the C–C bonds into oxygenated functionalities.39 The intensity ratio between the D-band and the G-band (ID/IG) is shown in Table 1 and Fig. SI3a.† In this particular case, the H2O2 treatment did not significantly affect the ID/IG ratio. This is in agreement with the results of Miyata et al. who also did not observe any change in the ID/IG ratio.18 However, these authors used a purified nanotube sample before the H2O2 treatment,24 which differs from the procedure reported here. In the G-band region from 1500 to 1650 cm−1 we can see two peaks in the Raman spectra of Fig. 2b, labeled here as the G+ and G− peaks centered at cm−1 >1590 cm−1 and <1590 cm−1, respectively (see Fig. 2b). The G+ peak is associated with carbon atoms vibrations along the axis of the carbon nanotube, whereas the G− intensity corresponds to carbon atoms on the outer, larger diameter tube of the DWNT.40 The G− shape is sensitive to semiconducting tubes with Lorentzian line shapes as well as to metallic tubes with Breit–Wigner–Fano line shapes.40 The G− region is also dependent upon dt, in which the smaller diameter CNTs exhibit a lower Raman frequency shift.41 The shape of the G band depends upon which CNT layer is in resonance at a given value of Elaser.42 For the DWNT–H2O2 tubes, the intensity of the G− shoulder (above 1550 cm−1) is slightly increased when excited by the laser of 1.69 eV. However, the G− shoulder below 1546 cm−1 exhibits a lower intensity at the 2.33 eV laser energy excitation, in agreement with the overall decrease of the intensity observed for the inner nanotubes, with regard to their RBM vibrations measured in this study. Changes in the intensity of the G− region could be due to charge-transfer in the Lorentzian and the Breit–Wigner–Fano line shapes,43 and the changes may come from oxidation effects, which are observed by comparing spectra for the pristine and H2O2 treated tubes.
| I D/IG | G′1 (cm−1) | G′2 (cm−1) | G′3 (cm−1) | G′4 (cm−1) | |
|---|---|---|---|---|---|
| a I D/IG is the ratio of the D-band and G-band peak intensity. The G′-band was deconvoluted into four peaks where G′1, G′2, G′3, and G′4. Furthermore, G′1 and G′2 correspond to the inner walls and G′3 and G′4 correspond to the outer walls of the DWNTs. | |||||
| DWNT | 0.037 | 2627 | 2648 | 2665 | 2692 |
| DWNT–H2O2 | 0.038 | 2638 | 2662 | 2676 | 2701 |
SWNTs exhibit two G′-band peaks for an individual SWNT, and these arise from double resonance processes. In addition, the position of these peaks can depend on the tube diameter; small diameter tubes exhibit a lower Raman G′ frequency shift and larger diameter tubes present a higher Raman frequency shift in the G′-band spectra.40,42,43 Therefore, for DWNTs one can expect four peaks, two of them corresponding to the inner nanotubes and the other two for the outer tubes.32 At higher Elaser the G′ peaks are more separated and it becomes easier to analyze the G′ spectra from DWNTs. For this reason we used Elaser = 2.33 eV to analyze the details of the G′ lineshape.32 From the G′ peak of DWNT–H2O2 (see Fig. 2c), the two deconvoluted peaks at lowest frequency exhibit a lower intensity than those for the pristine DWNT. This suggests that a decreased contribution comes from the signal of small diameter nanotube walls, and this interpretation is in accordance with the observations from the RBM and G-band from this sample.
In order to confirm the observations obtained by Raman spectroscopy for semiconducting nanotubes, the same methodology was applied to pure metallic (M-SWNT) and to pure semiconducting (S-SWNT) single-walled carbon nanotubes (see Fig. SI4 and SI5†). From these observations, it was found that semiconducting nanotubes exhibit a greater change in the RBM line shape when compared with metallic SWNTs. Small angle X-ray diffraction (XRD) was performed on the pristine and H2O2 treated DWNT fiber (see Fig. SI6†). The XRD results that were thus obtained exhibited two peaks: one broad peak at 4.9° 2θ was assigned to the (002) graphitic plane of the DWNT and for the amorphous carbon, and another peak at 9.2° 2θ which can be assigned to the (110) plane of the iron catalyst. After the H2O2 treatment the 9.2° 2θ peak intensity is decreased, suggesting the removal of the iron catalyst via the Fenton reaction with H2O2.29,30
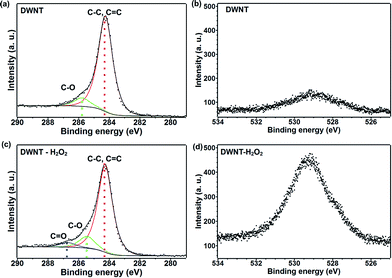
X-ray photoelectron spectroscopy (XPS) was carried out in order to elucidate the surface chemical composition of the fiber before and after the H2O2 treatment. In the C 1s peak, components with binding energy corresponding to sp2 hybridization was observed at 284.3 eV and a smaller contribution corresponding to sp3 hybridized carbon were observed within the same peak (see Fig. 3a).44,45 At 285.8 eV, the peak was assigned to C–O and a new peak located at 286.8 eV with low intensity arises after the H2O2 treatment, which corresponds to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalization (see Fig. 3c).29,46 This type of functionalization has been reported for H2O2 treatment on MWNTs, and may also be attributed to the oxidation of amorphous carbon due to its higher chemical reactivity.24,47 From the semi-quantitative elemental analysis, the oxygen content increases from 3.96 at% to 13.63 at% (see Fig. 3b and d and Table SI1†), due to the mild oxidation of the fibers. In addition, a smaller amount of iron was still present after the H2O2 treatment.
O functionalization (see Fig. 3c).29,46 This type of functionalization has been reported for H2O2 treatment on MWNTs, and may also be attributed to the oxidation of amorphous carbon due to its higher chemical reactivity.24,47 From the semi-quantitative elemental analysis, the oxygen content increases from 3.96 at% to 13.63 at% (see Fig. 3b and d and Table SI1†), due to the mild oxidation of the fibers. In addition, a smaller amount of iron was still present after the H2O2 treatment.
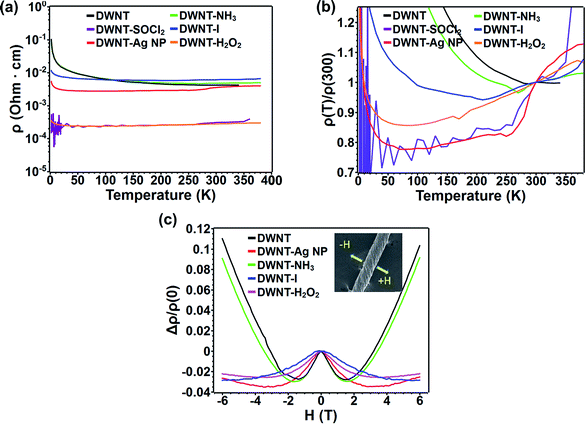
The electrical transport measurements of the fibers were carried out using a physical property measurement system (PPMS). Interestingly, the room temperature resistivity of the pristine DWNT fibers was found to be ca. 4.19 × 10−3 Ω cm and for DWNT–H2O2 the value decreased to 2.77 × 10−4 Ω cm, more than an order of magnitude lower than that of the pristine fiber. The H2O2 treatment was repeated on five different DWNT fibers from different synthesis batches and the average obtained resistivity value after treatment is 4.44 × 10−4 Ω cm and values vary ±3 × 10−4 Ω cm. The resistivity value obtained here is lower than that reported by Wei et al. who have also treated DWNTs with H2O2; however, Wei et al. used a film morphology12 in comparison to our nanotube fiber morphology. This difference in sample electrical resistivity could be caused by the promotion of a better wetting during our H2O2 treatment, which is consistent with our much larger expansion of the fiber during the H2O2 treatment. Furthermore, our DWNTs–H2O2 behave like a metal at temperatures above 100 K due to the decrease in electrical resistivity with decreasing temperature, and at lower temperatures the sample behaves like a semiconductor with increasing resistivity while lowering the temperature (see Fig. 4a and b). However, untreated DWNTs behave like a semiconductor over the entire temperature region. Our data (Fig. 4 and SI7†) were fitted according to the variable range hopping (VRH) model ρ(T) = ρ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−T0/T]1/(d+1) for d = 1, 2 and 3.48 Interestingly, before the H2O2 treatment, the DWNTs fit better for d = 1 and after the H2O2 treatment the fit is better for d = 3. These results were obtained by fitting the best R2 value for the linear regression fittings applied to each d value (see Fig. SI7 and Table SI2†). The normalized resistivity value at 2 K (ρ(2 K)/ρ(300 K)) decreased from 24.23 to 1.16 for DWNT–H2O2. The transverse magnetoresistance (MR%) measurements (see Fig. 4c) revealed that in the low magnetic field regime (<1.5 T), all samples exhibited negative MR%, with a H1/2 dependency, which is an indication of weak localization, as has been observed for SWNTs.49,50 At higher magnetic fields (>1.5 T) the DWNT sample followed a positive MR% as H2, which could be attributed to a spin-dependent variable range hopping conduction mechanism (see Fig. 4c). The DWNT–H2O2 sample preserves a negative MR% throughout the measured magnetic fields (−6 T to 6 T) (see Fig. 4c), and a similar behavior has been observed for strongly p-doped SWNTs.16 In order to compare the effects of H2O2 with other intercalates, DWNT fibers were also treated with: thionyl chloride (DWNT–SOCl2), silver nanoparticles (DWNT–Ag NP), ammonium hydroxide (DWNT–NH3) and iodine (DWNT–I) (see Fig. 4a and b). For DWNT–SOCl2 and DWNT–NH3, shorter times were used due to the complete decomposition of the fiber morphology after prolonged sonication. We noted that all of these agents could significantly decrease the resistivity, except for DWNT–NH3 which has been chosen in pursuit of possible n-type doping. For example, DWNTs–SOCl2 and DWNTs–I exhibit low electrical resistivity values in the order of 10−4 Ω cm, in which DWNT–SOCl2 exhibits the lowest value of 3.29 × 10−4 Ω cm, close to that of DWNT–H2O2 (see Table 2). The treatments with iodine, thionyl chloride and hydrogen peroxide showed an increase in fiber density after the final twisting step (see Table 2). This suggests that the intercalants and functionalization may well help to densify the fibers, thus decreasing the electrical resistivity. All the agents used (except for ammonium hydroxide) caused the DWNT fibers to behave as metals at temperatures above 200 K (see Fig. 4b). It might be possible that the metallic-like nanotubes dominate the electrical transport at high temperatures due to the increase in ohmic contact among the CNTs by an increase in fiber density and the removal of small diameter CNTs which could exhibit a larger band gap.35,51 At lower temperatures the structural defects (i.e. vacancies and oxidation-related groups) and intertube coupling give rise to charge localization effects that give the nanotubes a semiconductor behavior.52 We omitted the effect of H2O2 doping, because of the drying step after H2O2 treatment and the need of heating the fibers at 100 °C within the PPMS, when we measured the electric transport. All the p-type doping agents exhibit a negative MR, and the DWNT–NH3 sample displays a positive MR, at 2 K (see Fig. 4c and Table 2). These comparisons confirm that high electrical conductivities could be achieved with an environmentally friendly chemical such, as H2O2.
exp[−T0/T]1/(d+1) for d = 1, 2 and 3.48 Interestingly, before the H2O2 treatment, the DWNTs fit better for d = 1 and after the H2O2 treatment the fit is better for d = 3. These results were obtained by fitting the best R2 value for the linear regression fittings applied to each d value (see Fig. SI7 and Table SI2†). The normalized resistivity value at 2 K (ρ(2 K)/ρ(300 K)) decreased from 24.23 to 1.16 for DWNT–H2O2. The transverse magnetoresistance (MR%) measurements (see Fig. 4c) revealed that in the low magnetic field regime (<1.5 T), all samples exhibited negative MR%, with a H1/2 dependency, which is an indication of weak localization, as has been observed for SWNTs.49,50 At higher magnetic fields (>1.5 T) the DWNT sample followed a positive MR% as H2, which could be attributed to a spin-dependent variable range hopping conduction mechanism (see Fig. 4c). The DWNT–H2O2 sample preserves a negative MR% throughout the measured magnetic fields (−6 T to 6 T) (see Fig. 4c), and a similar behavior has been observed for strongly p-doped SWNTs.16 In order to compare the effects of H2O2 with other intercalates, DWNT fibers were also treated with: thionyl chloride (DWNT–SOCl2), silver nanoparticles (DWNT–Ag NP), ammonium hydroxide (DWNT–NH3) and iodine (DWNT–I) (see Fig. 4a and b). For DWNT–SOCl2 and DWNT–NH3, shorter times were used due to the complete decomposition of the fiber morphology after prolonged sonication. We noted that all of these agents could significantly decrease the resistivity, except for DWNT–NH3 which has been chosen in pursuit of possible n-type doping. For example, DWNTs–SOCl2 and DWNTs–I exhibit low electrical resistivity values in the order of 10−4 Ω cm, in which DWNT–SOCl2 exhibits the lowest value of 3.29 × 10−4 Ω cm, close to that of DWNT–H2O2 (see Table 2). The treatments with iodine, thionyl chloride and hydrogen peroxide showed an increase in fiber density after the final twisting step (see Table 2). This suggests that the intercalants and functionalization may well help to densify the fibers, thus decreasing the electrical resistivity. All the agents used (except for ammonium hydroxide) caused the DWNT fibers to behave as metals at temperatures above 200 K (see Fig. 4b). It might be possible that the metallic-like nanotubes dominate the electrical transport at high temperatures due to the increase in ohmic contact among the CNTs by an increase in fiber density and the removal of small diameter CNTs which could exhibit a larger band gap.35,51 At lower temperatures the structural defects (i.e. vacancies and oxidation-related groups) and intertube coupling give rise to charge localization effects that give the nanotubes a semiconductor behavior.52 We omitted the effect of H2O2 doping, because of the drying step after H2O2 treatment and the need of heating the fibers at 100 °C within the PPMS, when we measured the electric transport. All the p-type doping agents exhibit a negative MR, and the DWNT–NH3 sample displays a positive MR, at 2 K (see Fig. 4c and Table 2). These comparisons confirm that high electrical conductivities could be achieved with an environmentally friendly chemical such, as H2O2.
| Sample | Resistivityb (Ω cm) | MRc | Density (mg mm−3) | Specific conductivity (S m2 kg−1) |
|---|---|---|---|---|
| a Results are given for pristine (DWNTs), DWNTs with silver nanoparticles (DWNT–Ag NP), DWNT soaked with NH3 (DWNT–NH3) and with SOCl2 (DWNT–SOCl2), DWNTs intercalated with iodine (DWNT–I) and H2O2 treated (DWNT–H2O2). b Resistivity at 300 K. c MR magnetoresistance at 2 K. | ||||
| DWNT | 4.19 × 10−3 | Positive | 0.21 | 113.33 |
| DWNT–SOCl2 | 3.29 × 10−4 | Negative | 1.15 | 263.88 |
| DWNT–Ag NP | 3.52 × 10−3 | Negative | 0.10 | 268.41 |
| DWNT–NH3 | 4.78 × 10−3 | Positive | 0.58 | 35.98 |
| DWNT–I | 6.10 × 10−4 | Negative | 1.05 | 186.61 |
| DWNT–H2O | 6.29 × 10−3 | Positive | 0.56 | 28.11 |
| DWNT–H2O2 | 2.77 × 10−4 | Negative | 1.29 | 278.70 |
The samples were kept at room conditions for two months and the measured resistivity values exhibited a small resistivity increase to 4.307 × 10−4 Ω cm from 2.77 × 10−4 Ω cm. To rule out the effect of water adsorption, another fresh DWNT sample was sonicated in pure H2O; a resistivity of ca. 6.29 × 10−3 Ω cm was measured. The twisting effect was tested on a pristine DWNT fiber soaked in acetone. The fiber was fixed at both ends to a pair of clamps, and the resistivity was monitored after several turns or twists (see Fig. SI8†). The initial density of the fiber was 0.26 g cm−3, and the density changed to 0.53 g cm−3 after 60 turns (2.04 times higher), exhibiting a decrease in resistivity from 3.9 × 10−3 Ω cm to 2.4 × 10−3 Ω cm (1.62 times lower). Following the H2O2 treatments, the DWNT density changed from 0.21 g cm−3 to 1.29 g cm−3 (6.14 times higher), and the electrical resistivity changed from 4.19 × 10−3 Ω cm to 2.77 × 10−4 Ω cm (15.12 times lower). These results confirm that the fiber densification is also responsible for decreasing the electrical resistivity, along with the removal of small diameter CNTs that are predominantly semiconductors.
The following mechanism is proposed in order to explain the decrease in the electrical resistivity of DWNT fibers. The analysis of the G-band and the G′-band data indicated the removal of small diameter carbon nanotubes, by a decrease in the intensity of the first two deconvoluted peaks in the G′-band observed when using a 532 nm excitation laser (Fig. 2c). The higher oxidation reactivity towards smaller diameter carbon nanotubes has been observed by Zhou et al. for SWNTs, and a tendency was found for the presented H2O2 treatment.53 There is a very low possibility that oxidation could have removed small diameter CNTs during laser irradiation from the Raman measurement,25,37 because the inner wall tubes are protected by the outer wall tubes and residual H2O2 could have been removed during the drying process. XPS analysis revealed an increase in the oxygen content, which could be in the form of hydroxyl functional groups attached to the outer tubes. Whilst the hydrogen peroxide may decompose amorphous carbon and may later react with the iron nanoparticles following the Fenton reaction,29 thus lowering the iron content almost by half when compared with the pristine sample.
In addition, the degree of purification is less than that reported by Wei et al. (see Fig. 1e and h and SI1†), who have observed a decrease in electrical resistivity by one order of magnitude after DWNT purification.12 Therefore, our purification method may only affect in a small way the degree of decrease of the electrical resistivity. In this context, we noted that after sonicating the DWNT fibers in the presence of pure H2O there is no decrease in electrical resistivity (see Table 2). The twisting experiments demonstrate that increasing the fiber density could slightly decrease the electrical resistivity by allowing better electrical contact points among the DWNTs with each other (see Fig. SI8†). A complementary experiment consisting of the sonication in pure H2O indicated that the H2O2 treatment could have an etching effect with narrow diameter tubes that are more reactive. Because DWNT synthesis tends to yield more semiconducting than metallic CNTs, the removed small diameter nanotubes are mostly semiconducting, as observed by Raman spectroscopy (see Fig. 2); a more metallic sample was observed by the resistivity vs. temperature measurements (see Fig. 4), which is in agreement with the observations from Lekawa-Raus et al. who found similar trends in resistance-temperature dependence for CNT fibers with high metallic SWNT content.54
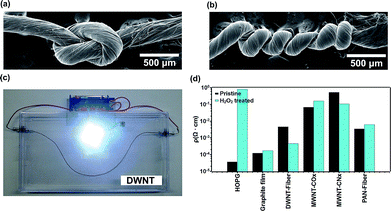
The mechanical properties of DWNT fibers were also measured, and the Young moduli of 4.14 MPa and 1.44 MPa were recorded for the pristine and H2O2 treated fibers, respectively. The tensile strength decrease from 50 MPa to 15 MPa, is possibly due to the formation of partial DWNT ruptures caused by the H2O2 treatment. Despite these relatively low tensile strength values, it was possible to handle and manipulate DWNT fibers, with the possibility of even making knots and twists with them (see Fig. 5a and b). Therefore, we believe that it is possible to use these fibers as electrical conducting cables. In order to illustrate this application, a working lighting device was assembled with a battery and a light emitting diode (LED) (see Fig. 5c). In order to demonstrate the versatility of the H2O2 treatment, we also sonicated other forms of carbon in H2O2, such as highly oriented pyrolytic graphite (HOPG), a graphite film, a DWNT fiber, MWNTs made by toluene/ethanol/ferrocene (MWNT–COx),27 nitrogen doped MWNTs (MWNT–CNx)28 and carbon fibers (PAN-fiber) (see Fig. 5d). The materials that already exhibit resistivity values of ca. 10−4 or lower (HOPG and the graphite film, without twisting) did not show any further decrease in resistivity after H2O2 treatment. MWNT–COx film (without twisting) initially exhibited an electrical resistivity of 6.61 × 10−2 Ω cm and the value is increased to 1.58 × 10−1 Ω cm after the H2O2 treatment, perhaps due to a severe oxidation during the treatment.39 For MWNT–CNx film (without twisting), the resistivity decreased from 0.514 Ω cm to 0.107 Ω cm, and in this scenario it is possible that the nitrogen sites may catalyze the decomposition of H2O2. This special case involves different interactions and will be published elsewhere. Carbonized PAN-fibers exhibited an increase of electrical resistivity from 3.41 × 10−3 Ω cm to 6.09 × 10−3 Ω cm, possibly due to the oxidation occurring at defect sites present within the material. DWNTs in the form of a fiber exhibit the largest change in resistivity (from 4.19 × 10−3 Ω cm to 3.77 × 10−4 Ω cm), which may be due to the particular morphology that favors exohedral doping, and its special chemistry and degree of crystallinity within the DWNT fibers. Wei et al. studied DWNTs in the form of films which could have inhibited a good H2O2 wetting within the bulk sample12 in the current case, the fiber de-bundling during the sonication suggested wetting with H2O2. In addition, the densification of the fiber after H2O2 treatment could result in a higher electrical percolation.
Conclusions
In summary, the H2O2 treatment significantly decreased the electrical resistivity of DWNT fibers. A careful analysis of the Raman spectra, XPS and electrical transport data demonstrated that the H2O2 treatment results in the preferential removal of small diameter semiconducting CNTs. These processes are favored in the bundle structure and are observed by an increase in volume within the solution. Furthermore, an increase in fiber density could further increase the electrical contact among the DWNTs. The method used in this work could yield low resistivity metallic-like DWNT fibers with low cost and a facile implementation method, with similar results to those obtained using other hazardous chemical agents. The use of H2O2 treated DWNT fibers can be helpful in promoting the use of CNTs in industry where highly conducting and lightweight materials are required (e.g. power transmission lines). In addition, these low resistivity CNTs could be used in conjunction with ceramics, polymers, electronic devices, medicine, among many others.Acknowledgements
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO). MT, SMVD, RCS, FTL and ME acknowledge support from the Research Center for Exotic Nanocarbons, Japan regional Innovation Strategy Program by the Excellence, JST. YAK acknowledges the financial support from the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2014R1A2A1A10050585). The synchrotron radiation experiments were performed at the BL04B2 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2013A1137). MSD acknowledges support from NSF/DMR 10-04147.Notes and references
- A. Oberlin, M. Endo and T. Koyama, J. Cryst. Growth, 1976, 32, 335 CrossRef CAS.
- M. Endo, Chem. Tech., 1988, 18, 568 CAS.
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- S. Iijima and T. Ichihashi, Nature, 1993, 363, 603 CrossRef CAS.
- E. T. W. Ebbesen, H. J. Lezec, H. Hiura, W. Bennett, H. F. Ghaemi and T. Thio, Nature, 1996, 382, 54 CrossRef.
- H. Dai, E. W. Wong and C. M. Lieber, Science, 1996, 272, 523 CAS.
- E. W. Wong, P. E. Sheehan and C. M. Lieber, Science, 1997, 277, 1971 CrossRef CAS.
- A. Thess, R. Lee, P. Nikolaev, H. Dai, P. Pierre, J. Robert, C. Xu, Y. H. Lee, S. G. Kim, A. G. Rinzler, D. T. Colbert, G. E. Scuseria, D. Tománek, J. E. Fischer and R. E. Smalley, Science, 1996, 273, 483 CAS.
- J. E. Fischer, H. Dai, A. Thess, R. Lee, N. M. Hanjani, D. L. Dehaas and R. E. Smalley, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 4921 CrossRef.
- H. W. Zhu, C. L. Xu, D. H. Wu, B. Q. Wei, R. Vajtai and P. M. Ajayan, Science, 2002, 296, 884 CrossRef CAS PubMed.
- Y. Zhao, J. Wei, R. Vajtai, P. M. Ajayan and E. V. Barrera, Sci. Rep., 2011, 1, 83 Search PubMed.
- J. Wei, H. Zhu, B. Jiang, L. Ci and D. Wu, Carbon, 2003, 41, 2495 CrossRef CAS.
- G. Chen, D. N. Futaba, S. Sakurai, M. Yumura and K. Hata, Carbon, 2014, 67, 318 CrossRef CAS.
- T. Enoki, M. Suzuki and M. Endo, in Graphite Intercalation Compounds and Applications, Oxford University Press, Oxford, UK, 2003 Search PubMed.
- B. Ruzicka, L. Degiorgi, R. Gaal, L. Thien-Nga, R. Bacsa, J.-P. Salvetat and L. Forro, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 2468 CrossRef.
- R. S. Lee, H. J. Kim, J. E. Fischer, A. Thess and R. E. Smalley, Nature, 1997, 388, 255 CrossRef CAS.
- H. Tantang, J. Y. Ong, C. L. Loh, X. Dong, P. Chen, Y. Chen, X. Hu, L. P. Tan and L.-J. Li, Carbon, 2009, 47, 1867 CrossRef CAS.
- Y. Miyata, K. Yanagi, Y. Maniwa and H. Kataura, J. Phys. Chem. C, 2008, 112, 3591 CAS.
- V. Skákalová, A. B. Kaiser, U. Dettlaff-Weglikowska, K. Hrnčariková and S. Roth, J. Phys. Chem. B, 2005, 109, 7174 CrossRef PubMed.
- U. Dettlaff-Weglikowska, V. Skákalová, R. Graupner, S. H. Jhang, B. H. Kim, H. J. Lee, L. Ley, Y. W. Park, S. Berber, D. Tománek and S. Roth, J. Am. Chem. Soc., 2005, 127, 5125 CrossRef CAS PubMed.
- N. Behabtu, C. C. Young, D. E. Tsentalovich, O. Kleinerman, X. Wang, A. W. Ma, E. A. Bengio, R. T. Waarbeek, J. J. Jong, R. E. Hoogerwerf, S. B. Fairchild, J. B. Ferguson, B. Maruyama, J. Kono, Y. Talmon, Y. Cohen, M. J. Otto and M. Pasquali, Science, 2013, 339, 182 CrossRef CAS PubMed.
- R. Graupner, J. Abraham, A. Vencelová, T. Seyller, F. Hennrich, M. M. Kappes, A. Hirsch and L. Ley, Phys. Chem. Chem. Phys., 2003, 5, 5472 RSC.
- J. D. Wiggins-Camacho and K. J. Stevenson, J. Phys. Chem. C, 2009, 113, 19082 CAS.
- Y. Miyata, Y. Maniwa and H. Kataura, J. Phys. Chem. B, 2006, 110, 25 CrossRef CAS PubMed.
- M. Yudasaka, M. Zhang and S. Iijima, Chem. Phys. Lett., 2003, 374, 132 CrossRef CAS.
- S. Coskun, B. Aksoy and H. E. Unalan, Cryst. Growth Des., 2011, 11, 4963 CAS.
- A. Botello-Méndez, J. Campos-Delgado, A. Morelos-Gómez, J. M. Romo-Herrera, A. G. Rodríguez, H. Navarro, M. A. Vidal, H. Terrones and M. Terrones, Chem. Phys. Lett., 2008, 453, 55 CrossRef.
- M. Terrones, R. Kamalakaran, T. Seeger and M. Rühle, Chem. Commun., 2000, 2335 RSC.
- K. V. Voitko, R. L. D. Whitby, V. M. Gun'ko, O. M. Bakalinska, M. T. Kartel, K. Laszlo, A. B. Cundy and S. V. Mikhalovsky, J. Colloid Interface Sci., 2011, 361, 129 CrossRef CAS PubMed.
- K. R. Moonoosawmy and P. Kruse, J. Am. Chem. Soc., 2010, 132, 1572 CrossRef CAS PubMed.
- J. Wei, B. Jiang, X. Zhang, H. Zhu and D. Wu, Chem. Phys. Lett., 2003, 376, 753 CrossRef CAS.
- F. Villalpando-Paez, H. Son, S. G. Chou, G. G. Samsonidze, Y. A. Kim, H. Muramatsu, T. Hayashi, M. Endo, M. Terrones and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 035419 CrossRef.
- A. Jorio, C. Fantini, M. A. Pimenta, R. B. Capaz, G. G. Samsonidze, G. Dresselhaus, M. S. Dresselhaus, J. Jiang, N. Kobayashi, A. Grüneis and R. Saito, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 075401 CrossRef.
- M. Endo, Y. A. Kim, T. Hayashi, H. Muramatsu, M. Terrones, R. Saito, F. Villalpando-Paez, S. G. Chou and M. S. Dresselhaus, Small, 2006, 2, 1031 CrossRef CAS PubMed.
- H. Kataura, Y. Kumazawa, Y. Maniwa, I. Umezu, S. Suzuki, Y. Ohtsuka and Y. Achiba, Synth. Met., 1999, 103, 2555 CrossRef CAS.
- G. G. Samsonidze, R. Saito, N. Kobayashi, A. Grüneis, J. Jiang, A. Jorio, S. Chou, G. Dresselhaus and M. S. Dresselhaus, Appl. Phys. Lett., 2004, 85, 5703 CrossRef CAS.
- J.-Y. Mevellec, C. Bergeret, J. Cousseau, J.-P. Buisson, C. P. Ewels and S. Lefrant, J. Am. Chem. Soc., 2011, 133, 16938 CrossRef CAS PubMed.
- S. Osswald, E. Flahaut and Y. Gogotsi, Chem. Mater., 2006, 18, 1525 CrossRef CAS.
- Y. Peng and H. Liu, Ind. Eng. Chem. Res., 2006, 45, 6483 CrossRef CAS.
- M. S. Dresselhaus, G. Dresselhaus, R. Saito and A. Jorio, Phys. Rep., 2005, 409, 47 CrossRef.
- A. Jorio, A. G. Souza Filho, G. Dresselhaus, M. S. Dresselhaus, A. K. Swan, M. S. Ünlü, B. B. Goldberg, M. A. Pimenta, J. H. Hafner, C. M. Lieber and R. Saito, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 155412 CrossRef.
- F. Villalpando-Paez, H. Son, D. Nezich, Y. P. Hsieh, J. Kong, Y. A. Kim, D. Shimamoto, H. Muramatsu, T. Hayashi, M. Endo, M. Terrones and M. S. Dresselhaus, Nano Lett., 2008, 8, 3879 CrossRef CAS PubMed.
- A. G. Souza Filho, A. Jorio, G. G. Samsonidze, G. Dresselhaus, M. A. Pimenta, M. S. Dresselhaus, A. K. Swan, M. S. Ünlü, B. B. Goldberg and R. Saito, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 035427 CrossRef.
- W.-J. Chou, C.-C. Wang and C.-Y. Chen, J. Inorg. Organomet. Polym., 2009, 19, 234 CrossRef CAS.
- H. Ago, T. Kugler, F. Cacialli, W. R. Salaneck, M. S. P. Shaffer, A. H. Windle and R. H. Friend, J. Phys. Chem. B, 1999, 103, 8116 CrossRef CAS.
- S. Kundu, Y. Wang, W. Xia and M. Muhler, J. Phys. Chem. C, 2008, 112, 16869 CAS.
- J. T. Han, S. Y. Kim, J. S. Woo, H. J. Jeong, W. Oh and G.-W. Lee, J. Phys. Chem. C, 2008, 112, 15961 CAS.
- B. I. Shklovskii and A. L. Efros, Electronic Properties of Doped Semiconductors, In Springer Series in Solid-State Sciences, ed. M. Cardona, P. Fulde and H.-J. Queisser, Springer, Berlin, 1984 Search PubMed.
- J. Vavro, J. M. Kikkawa and J. E. Fischer, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 155410 CrossRef.
- G. T. E. Kim, S. Choi, D. C. Kim, D. S. Suh, Y. W. Park, K. Liu, G. Duesberg and S. Roth, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 16064 CrossRef CAS.
- J. N. Wang, X. G. Luo, T. Wu and Y. Chen, Nat. Commun., 2014, 5, 3848 CAS.
- J. M. Marulanda, Carbon Nanotubes, InTech, Vukovar, 2010, p. 123 Search PubMed.
- W. Zhou, Y. H. Oou, R. Russo, P. Papanek, D. E. Luzzi, J. E. Fischer, M. J. Bronikowski, P. A. Willis and R. E. Smalley, Chem. Phys. Lett., 2001, 350, 6 CrossRef CAS.
- A. Lekawa-Raus, K. Walczak, G. Kozlowski, M. Wozniak, S. C. Hopkins and K. K. Koziol, Carbon, 2015, 84, 118 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ta06662j |
| ‡ The current address of AMG, RCS and II is Global Aqua Innovation Center. |
| This journal is © The Royal Society of Chemistry 2016 |