MoS2 supported single platinum atoms and their superior catalytic activity for CO oxidation: a density functional theory study†
Abstract
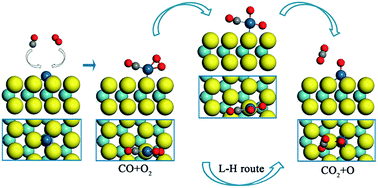
Late transition metals, such as Rh, Ir, Pd and Pt, have a strong tendency to form a square-planar 16-electron complex. Although this feature has been widely used in organometallics to develop homogeneous catalysts, a single-atom heterogeneous analogue has not yet been reported. In this work, we show that a 16-electron complex may act as an important transition state in the CO oxidation over a single Pt atom supported by a MoS2 monolayer (Pt/MoS2). The catalytic oxidation reaction prefers to start with the Langmuir–Hinshelwood (L–H) reaction, where the CO and O2 molecules are first co-adsorbed on the Pt atom, then cross a small barrier of 0.40 eV to form a square-planar 16-electron intermediate state, and subsequently the first CO2 is released. The activation barrier of the following Eley–Rideal (E–R) reaction is only 0.23 eV. The superior catalytic reactivity of the Pt/MoS2 surface can be explained by the quantum confinement effect of the Pt-5d orbitals and the stability of the square-planar 16-electron transition state. In addition, MoS2 may serve as a defect-free two dimensional anchoring substrate for Pt atomic adsorption. It provides not only a very large surface-to-volume ratio, but also a well-defined structure with a uniform distribution of anchoring points. The square-planar 16-electron intermediate state of the L–H reaction, together with the new MoS2 anchoring substrate, may provide a new opportunity for the design of single-atom catalysts on two-dimensional surfaces.

 Please wait while we load your content...
Please wait while we load your content...