A “H2O donating/methanol accepting” platform for preparation of highly selective Nafion-based proton exchange membranes†
Abstract
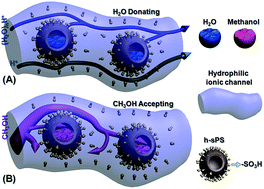
For a proton exchange membrane (PEM), the ratio of its proton conductivity to its fuel permeability usually defines the membrane selectivity. Generally, a highly selective PEM is preferred for application in direct methanol fuel cells. Herein, sulfonated SiO2@polystyrene core–shell (SiO2@sPS) nanoparticles were synthesized and then imbedded into a Nafion membrane by a blending–casting method. SiO2@sPS partakes in strong interactions with the Nafion polymer, which benefits its dispersion in the membrane matrix. The as-prepared SiO2@sPS + Nafion composite PEM presents a large increase in its proton conductivity owing to the introduction of additional –SO3H groups and hence has optimized channels for proton transport. Meanwhile, a reduced methanol crossover was also observed for the SiO2@sPS + Nafion composite PEM because of the formation of obstructed transport channels for bulk methanol. Besides this, a deep investigation on further enhancement of the membrane’s performance was conducted by etching the SiO2 core and hence forming well-dispersed uniform hollow spheres inside the membrane matrix. The intact hollow sulfonated PS spheres (h-sPS) acted as water reservoirs which in turn could gradually release water to hydrate the membrane under high-temperature and low-humidity conditions. Therefore, compared to the SiO2@sPS + Nafion membrane, the h-sPS + Nafion one presented a further increased proton conductivity at 100 °C under 40% RH. Meanwhile, h-sPS further suppressed methanol penetration by trapping it inside the hollow spheres. Herein, a “H2O donating/methanol accepting” mechanism was proposed for the first time, providing a promising platform to alleviate critical disadvantages of Nafion membranes and thereby fabricate highly selective Nafion-based PEMs.

 Please wait while we load your content...
Please wait while we load your content...