Influence of phase transformation pathways on electrochemical properties by using thermally derived solid-solution LiFePO4 nanoparticles†
Abstract
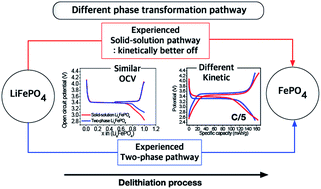
To understand how a phase transformation pathway affects the electrochemical properties of LiFePO4 nanoparticles (NPs), a sample with the solid-solution phase (SS sample) was prepared by thermal treatment of a sample that contained two phases, LiFePO4 and FePO4, (TP). The SS sample had NPs that experience the solid-solution phase and then may undergo solid-solution phase transformation, and the TP sample had NPs that do not experience and then can undergo typical two-phase transformation. And their electrochemical characteristics were compared under various conditions. The thermodynamic properties of the two samples were evaluated using a galvanostatic intermittent titration technique (GITT) and a potentiostatic intermittent titration technique (PITT), and electrochemical kinetic properties were evaluated by applying current. The two samples had quite similar thermodynamic properties such as OCV and diffusion coefficient, but quite different kinetic properties such as polarization, current decay, voltage flatness and underpotential behavior even though they had a similar particle size and size distribution. The SS sample showed lower polarization, faster current decay at a constant voltage and less-significant underpotential behavior than did the TP sample. Furthermore, during charging/discharging, the voltage profile was a slope for the SS sample but flat for the TP sample even though the OCV of the two samples did not show any significant difference. These facile electrochemical characteristics can be related to nucleation indicating that the SS sample can have less significant nucleation than the TP sample. These different electrochemical properties are caused by different phase transformation pathways rather than the particle size that is the typical cause for those different kinetic properties. The phase transformation pathway of LiFePO4 strongly affects the electrochemical kinetic properties, not the thermodynamic ones. We reveal that undergoing the solid-solution pathway can be kinetically better off leading to a fast electrochemical response.

 Please wait while we load your content...
Please wait while we load your content...