Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improving the mechanical stability of zirconium-based metal–organic frameworks by incorporation of acidic modulators†
Ben
Van de Voorde
a,
Ivo
Stassen
a,
Bart
Bueken
a,
Frederik
Vermoortele
a,
Dirk
De Vos
a,
Rob
Ameloot
a,
Jin-Chong
Tan
*b and
Thomas D.
Bennett
*c
aCentre for Surface Chemistry and Catalysis, KU Leuven, Arenbergpark 23, B-3001 Leuven, Belgium
bDepartment of Engineering Science, University of Oxford, Parks Road, Oxford OX1 3PJ, UK
cDepartment of Materials Science and Metallurgy, University of Cambridge, Cambridge CB3 0FS, UK. E-mail: tdb35@cam.ac.uk
First published on 1st December 2014
Abstract
The ability to retain structural integrity under processing conditions which involve mechanical stress, is essential if metal–organic frameworks (MOFs) are to fulfil their potential as serious candidates for use in gas sorption, separation, catalysis and energy conversion applications. A series of zirconium dicarboxylates, predicted to be amongst the more mechanically robust MOFs, have been found to undergo rapid collapse upon ball-milling, resulting in catastrophic losses of porosity. An inverse relationship between collapse time and framework porosity has been found. Addition of acidic modulator ligands (e.g. trifluoroacetic acid) to UiO-66 provided a striking increase in mechanical robustness, the degree of which is inversely related to modulator pKa. This effect, caused by an increased strength of the zirconium–carboxylate bond, provides an important concept to design microporous hybrid frameworks capable of retaining their structure under harsh processing conditions.
Introduction
Metal–organic frameworks (MOFs) are microporous materials incorporating both organic and inorganic moieties connected in a three-dimensional crystal lattice.1 Extensive research has been performed on the synthesis and structure of MOFs, with applications proposed in e.g. separation, gas storage, sensing and catalysis.2–6 Despite commercial and industrial applications of MOFs necessitating the retention of structure and associated porosity during shaping or post-synthetic processing of powders by mechanical pelletization, extrusion or sintering (forming a solid mass by heating), their physical properties are not well understood at present.7,8 The instability of some MOFs has already proven problematic in proof-of-concept practical applications,9–11 though it has been harnessed in other cases to create novel materials12,13 and also employed to effect the trapping of harmful species.14The zirconium-containing frameworks UiO-66 and the MIL-140 series (Fig. 1) possess chemical and thermal stabilities surpassing those of many other MOFs, which is ascribed to strong bonds between the carboxylates and high valent Zr.15,16
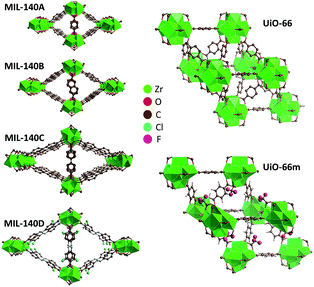 | ||
| Fig. 1 Structures of MIL-140 (A–D), UiO-66 and UiO-66m TFA, with cluster-capping trifluoroacetate molecules. | ||
This observation, combined with computational reports of the mechanical stability conferred to UiO-66 by its 12-fold connected rigid Zr6O4(OH)4 inorganic building blocks,15,17 renders them excellent candidates for future industrial applications.
Recent research has, however, suggested that crystal defects (e.g. missing linkers18) may play a key role in determining mechanical stability, and thus empirical verification of the predicted rigid elastic properties is needed. In the following work, controlled time-dependent ball-milling is used as a high-impact mechanical treatment to gauge stability in shaping operations. We show that, similar to other MOFs,21 Zr-containing frameworks are highly susceptible to mechanically-induced structural collapse. The inverse relationship between collapse time and micropore volume strongly suggests that the continuing search for higher surface area MOFs1,22 is unlikely to be accompanied with increases in mechanical strength, and methods for stabilizing these structures must therefore be developed.
Remarkably, addition of different acidic mono-coordinating ligands, which we term modulators, to the structural Zr6O4(OH)4 clusters resulted in striking increases in mechanical stability without alteration of the porosity. The stability increase of the modulated UiO-66 variants is enhanced with decreasing modulator pKa, and is explained by the generation of progressively stronger zirconium–carboxylate bonds through a local electron withdrawing effect. This electronic modulation concept may prove applicable to other MOFs and play a key role in synthesizing structures which are capable of withstanding the mechanical processing necessary for their use in industrial applications.
Experimental
Materials selection and synthesis
The four isoreticular variants of the Zr-oxo chain-based MIL-140 framework selected for this study contain one-dimensional pores with different widths, and were synthesized and evacuated according to previously described literature procedures.16 The three-dimensionally porous framework UiO-66 was synthesized in a Schott DURAN® pressure plus bottle with a volume of 1 l under static conditions, starting from an equimolar solution of ZrCl4 (3.5 g, 15 mmol) and terephthalic acid (2.5 g, 15 mmol) dissolved in DMF (N,N-dimethylformamide; 155 ml, 2 mol). 1.5 ml of a 36 wt% solution of HCl (17 mmol) was added to the solution and the mixture was placed in a preheated oven at 120 °C for 21 h. The powders were collected via centrifugation and thoroughly washed (3 times each) with DMF and ethanol.A series of UiO-66m X variants (X = TFA – trifluoroacetic acid, ClA – chloroacetic acid, AA – acetic acid) were also studied, being prepared by addition of the acid to the above mixture prior to heating. 10 equivalents (11.5 ml) of CF3COOH (TFA), 15 equivalents (21.26 ml) of ClH2COOH (ClA) or 50 equivalents (42.9 ml) of CH3COOH (AA) were added to the mixture in three separate reactions. The different concentrations of modulator were selected to obtain similar degrees of incorporation of modulator in all samples. For UiO-66m TFA, the addition of the modulator during the synthesis has previously been shown to result in partial replacement of bridging 1,4-benzenedicarboxylate linkers by cluster-capping TFA species.23 TGA analysis (Fig. S1†) confirmed that comparable quantities of modulator were present in the other modulated samples.
Zeolitic imidazolate framework ZIF-8 [Zn(mIm)2] (mIm: 2-methylimidazolate) was subjected to an identical milling treatment for comparison, and was activated after being purchased from Sigma Aldrich.
Evaluation of mechanical stability
Analysis of the kinetics of framework collapse was performed by monitoring the evolution of the integral breadth of the last remaining diffraction peak with milling time, in accordance with the method used for analyzing the crystallinity of UiO-66 frameworks under pressure.16 Collapse times were taken as the point at which no Bragg peaks were remaining.In all milling treatments, 50 mg of each sample was placed in a 10 ml stainless steel jar along with one 9 mm diameter stainless steel ball. The material was then ball-milled for varying amounts of time in a Retsch MM400 grinder mill operating at 20 Hz. Samples were not placed back into the ball-mill after analysis (i.e. 20 minutes of ball-milling was done continuously, rather than four 5 minute intervals), to prevent mass loss during analysis. Temperature increases in previous ball-milling experiments have been observed to be negligible.24
Room temperature PXRD data were collected after milling, using a Bruker-AXSD8 diffractometer using CuKα1 (λ = 1.540598 Å) radiation and a LynxEye position sensitive detector in Bragg Brentano parafocusing geometry. Analysis of the data was carried out using the X'Pert HighScore Plus program. Structureless pattern profile refinements of low angle data (2θ = 5–20°) were also carried out using X'Pert HighScore Plus. Refinement of experimental background, cell parameters, W and V parameters and two asymmetry parameters were undertaken. The integral breadth (peak area/peak maximum) of the first diffraction peak at 2θ ≈ 7° (the last remaining peak upon amorphization) was used instead of the FWHM, as an anti-scattering knife-edge on the X-ray diffractometer resulted in an increasing background at low-angle. The last remaining diffraction peaks used for profile fitting were the (200), (111) and (011) for MIL-140 frameworks, the UiO-66 variants and ZIF-8 respectively (Fig. S2a–l†).
Characterization of materials
Nitrogen physisorption isotherms were measured at 77 K with a Micromeritics 3Flex surface characterization analyser. Prior to the measurements, the powders (50–80 mg) were outgassed for 4 h at 473 K and 10−1 mbar vacuum.Surface area and pore size distribution calculations were carried out using the 3Flex Version 1.01 program. The BET method was applied on the data points between 0.005 and 0.05 p/p0. Calculation in this region resulted in both a positive C value and an increasing Rouquerol transform for all samples. External surfaces were calculated with the t-plot method (Harkins and Jura thickness equation; thickness range 3.5–5 Å). Micropore areas were obtained by subtracting the external surface from the BET surface area. Pore-size distributions were calculated with the Tarazona NLDFT model (regularization factor 0.001).
For IR spectroscopy, the samples (1 wt%) were pressed in a KBr pellet and were outgassed in the sample chamber under secondary vacuum (<4 mbar) at room temperature for the spectra of the 400–800 and 1600–1800 cm−1 regions, or at elevated temperatures (50–200 °C) for the 3500–3800 cm−1 region. Transmission IR spectra were measured on a FTIR Bruker type IFS 66/v/S equipped with a cryo-detector, with a 4 cm−1 resolution.
Thermogravimetric analyses were performed on a TA instruments TQA 500 machine. Measurements were performed by heating the samples at 5 °C min−1 under nitrogen.
Scanning electron micrographs were recorded on Au-coated samples using a Philips XL30 FEG microscope.
Results and discussion
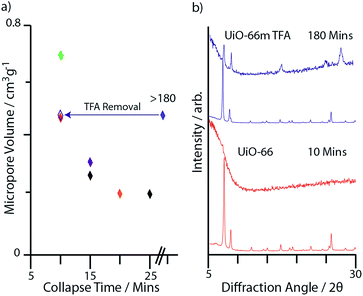
Facile, porosity-dependent collapse
Powder X-ray diffraction indicated rapid losses in crystallinity upon milling of the non-modulated frameworks, resulting in loss of Bragg intensity and appearance of broad diffuse scattering, indicating amorphization. For the MIL-140 frameworks, collapse times decreased with increasing linker length, whilst UiO-66 was the least stable Zr-MOF (Table 1). For this series of five Zr-MOFs, this results in an inversely proportional relationship between micropore volume and collapse time (Fig. 2a). The short collapse time of UiO-66 of less than 10 minutes is remarkably similar to that of the ‘soft’ porous framework ZIF-8, which contains relatively simple ZnN4 tetrahedral inorganic nodes. Indeed, the comparable pore volumes of the two materials (Table 1) suggest that no significant stabilization against ball-milling is conferred to UiO-66 by the multi-connected nature of its Zr6O4(OH)4 building block. This is surprising given the differences between the two structures and recent theoretical studies on UiO-66 suggesting its particularly robust nature.17| Name | Ideal structural formula | Measured micropore volumea (cm3 g−1) | Collapse time/min | Minimum and maximum shear moduli Gmin/Gmaxb (GPa) |
|---|---|---|---|---|
| a Total pore volume determined at p/p0 = 0.9. b Theoretical predictions obtained from density functional theory (DFT)17,19 except for ZIF-8 (values derived from Brillouin scattering experiments20). c Based on TGA analysis, the actual formula in this study is Zr6O6(OH)2[O2C–C6H4–CO2]5. C6H4 – benzene, C10H6 – naphthalene, C12H8: 4,4′-biphenyl. | ||||
| MIL-140 A | ZrO[O2C–C6H4–CO2] | 0.218 | 20–25 | 0.65/23.4 (ref. 19) |
| MIL-140 B | ZrO[O2C–C10H6–CO2] | 0.214 | 15–20 | |
| MIL-140 C | ZrO[O2C–C12H8–CO2] | 0.280 | 10–15 | |
| MIL-140 D | ZrO[O2C–C12H6Cl2–CO2] | 0.322 | 10–15 | |
| UiO-66 | Zr6O4(OH)4[O2C–C6H4–CO2]6c | 0.482 | 5–10 | 13.75/17.63 (ref. 17) |
| ZIF-8 | Zn(C4H5N2)2 | 0.694 | 5–10 | 0.94/1.33 (ref. 20) |
Low values of the minimum shear modulus, Gmin, of MOFs have previously been invoked to explain their instability and in particular, the rapid collapse of ZIF-8 (Gmin < 1 GPa).20 Intriguingly, the considerably higher theoretical shear modulus of UiO-66 (13.75 GPa), which is nearly isotropic (Gmax/Gmin = 1.28),17 does not lead to an increase in mechanical stability. One might also expect MIL-140 A (Gmin = 0.65 GPa) to exhibit a low resilience to collapse; however, it is unexpectedly more robust than both UiO-66 and ZIF-8. One possible explanation is that for MIL-140 A, the amorphization process is slowed down by the relatively large predicted Gmax,19 in combination with short-range dispersive interactions arising from π–π stacking of the aromatic linkers.16 However, the presence of defects in materials like UiO-66 may also strongly influence the eventual mechanical properties,18 and this raises the question how materials might be modified to reach their theoretical stabilities.
The reduction in particle size of each sample upon ball-milling was monitored by scanning electron microscopy (SEM). Initial crystallite sizes ranged from 2–10 µm for the MIL-140 series, to <1 µm for the UiO-66 variants (Fig. S3a–g†). Rapid decreases in particle size were noted during the first ten minutes of milling, with no significant further changes. The dissociation between particle size and crystallinity is clearly observed for MIL-140 A–C, which retain crystallinity upon reduction to sub-micron particle sizes (Fig. S2a–c†), and then undergo subsequent loss of long range order (amorphization) with negligible further changes in particle size.
Acidic modulator based framework stabilization
Recently, the incorporation of trifluoroacetic acid in the UiO-66 framework has been reported to assist in the generation of additional Lewis acid sites and to increase the catalytic activity.23 TFA groups partly replace some of the cluster linking benzene dicarboxylate ligands in this material (UiO-66m TFA), leading to a more open framework with a representative formula Zr6O7(OH)[O2C–C6H4–CO2]4[CF3CO2]. The TFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratio of 10
Zr ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 used in the literature synthesis results in an average incorporation of one trifluoroacetate (TFA) per cluster as determined by solid state 19F-NMR.23
1 used in the literature synthesis results in an average incorporation of one trifluoroacetate (TFA) per cluster as determined by solid state 19F-NMR.23
Notably, despite possessing almost the same micropore volume as UiO-66 (0.497 vs. 0.492 cm3 g−1), UiO-66m TFA exhibited remarkable stability upon ball-milling, retaining Bragg diffraction for over 3 hours (Fig. 2b and S2g†). This is in strong contrast to previous studies on the ZIF series, where higher elastic moduli and thus framework rigidity are typically linked to increases in framework density.25 Upon heating at 350 °C for several hours, TFA molecules are removed from the UiO-66m TFA (Fig. S4a†).23 Samples treated as such, termed UiO-66mh, were observed to collapse in a similar time (<10 min) as the non-modulated UiO-66 sample (Fig. S2i†), providing evidence for the beneficial effect of the modulator in enhancing the mechanical stability of the framework.
The N2 gas uptake capacities at 77 K of all crystalline and amorphized frameworks (hereby referred as amMOFs, where the subscript m indicates amorphization by ball-milling) were measured, along with their BET, micropore and external surface areas (Table 2). In the analysis, the partially amorphized UiO-66m TFA after 25 minutes of ball-milling was used, to provide some comparison with the other materials.
| Sample | BET (m2 g−1) | Micropore (m2 g−1) | External area (m2 g−1) |
|---|---|---|---|
| MIL-140 A | 484 (16) | 425 (3) | 59 (13) |
| MIL-140 B | 483 (41) | 405 (29) | 78 (13) |
| MIL-140 C | 682 (23) | 601 (15) | 81 (7) |
| MIL-140 D | 545 (6) | 472 (1) | 73 (6) |
| UiO-66 | 1129 (13) | 1003 (3) | 126 (10) |
| UiO-66m TFA | 1249 (254) | 1152 (203) | 97 (51) |
| ZIF-8 | 2048 (43) | 2382 (10) | 23 (33) |
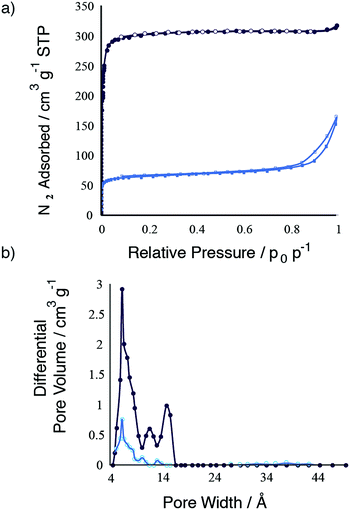
The type I isotherms for the crystalline phases (Fig. S5a†) are in broad agreement with those previously published.16 Unsurprisingly, overall BET surfaces decrease markedly upon amorphization in each case, accompanied by a striking decrease to negligible micropore volumes (Table 2). This was not the case for UiO-66m TFA, where N2 physisorption analysis (Fig. 3) indicated retention of ca. 20% of micropore volume upon ball-milling.
Pore size distributional analysis of crystalline UiO-66 (Fig. S5b–g†) illustrated not only the presence of the expected small tetrahedral and large octahedral cages; even larger cage and channel dimensions were found. We ascribe this to the now well-documented phenomenon of linker deficiency, resulting in the merger of neighbouring cages.18,23,26,27 TGA analysis showed ∼2 missing linkers per cluster for UiO-66. This phenomenon is even more pronounced for UiO-66m TFA (Fig. 3), in which partial substitution of terephthalate linkers by incorporated TFA molecules leaves only 8 bicipital linkers around the cluster, rather than the maximum of 12. After partial amorphization, the residual microporosity largely corresponds to the smallest pore diameters in the starting material, indicating that the largest pores in the starting UiO-66m TFA suffer the most drastic decreases in associated volume upon milling.
Structural changes upon milling
Samples of amMIL-140 A–D were observed to lose 5–10 wt% of water between 50 and 150 °C before thermal degradation at 500 °C (Fig. S6a†). This is in contrast to the negligible water loss for the crystalline frameworks, which are hydrophobic. The increased hydrophilicity upon amorphization is in agreement with an increase in the free carboxylic acid IR stretching vibration at ∼1700 cm−1, and the detection of strongly bound water for amorphized MIL-140 A (Fig. S7a†).Markedly different behavior is observed in the cases of crystalline UiO-66 and UiO-66m TFA, which show two weight loss steps corresponding to release of physisorbed H2O at 100 °C, and dehydroxylation of the Zr clusters around 250 °C. An additional step is present in the TGA for the latter at ca. 300 °C, corresponding to the loss of trifluoroacetate molecules, in agreement with existing literature.23 Initial weight loss from amUiO-66 and amUiO-66m TFA is much lower, reflecting a decrease in porosity relative to their parent crystalline frameworks. As the amorphization of UiO-66 is complete after 10 minutes of milling, no further increase in free carboxylate stretch can be detected even after prolonged milling (10–20 minutes).
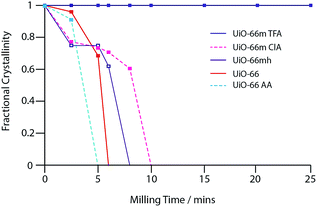
To understand the remarkable increase in mechanical stability of UiO-66m TFA, samples of UiO-66 modulated with acetic acid (UiO-66m AA) and chloroacetic acid (UiO-66m ClA)23 were also analysed (Fig. S2j and k†). Collapse times were found to be inversely related to the pKa value of the modulator or ligand, with UiO-66m TFA remaining the most stable sample (Table 3). Analysis of the increase in integral breadth of the last remaining diffraction peaks with milling time (Fig. 4) confirmed the intermediate stability of the sample modulated with chloroacetic acid.
The stabilization is ascribed to an electronic effect on the Zr–carboxylate bonds by the incorporation of the modulator, since its removal from UiO-66m TFA results in shorter collapse times. This can be understood as a local electron withdrawing effect, which increases the positive partial charge on the Zr4+ atom and resulting in a stronger ionic Zr–carboxylate bond.28,29
Experimental evidence for the effect is directly provided by IR spectroscopy (Fig. 5). The band at ca. 744 cm−1 (assigned to in phase C–H bending of the terephthalate) remains invariable for the different modulated materials. The band around 545–555 cm−1 is assigned to the Zr–(OC) asymmetric stretching vibration,26 and moves to progressively higher wavenumber with decreasing modulator pKa, implying a strengthening of the bond. The position of this band in UiO-66m AA and UiO-66 is consistent with the rapid collapse of these materials. Note that the symmetric stretching is not observed in hydroxylated UiO-66 samples.26
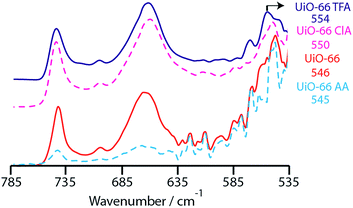 | ||
| Fig. 5 IR spectra following the Zr–OC asymmetric stretching vibration of the modulated UiO-66 samples. | ||
The vibration at 663 cm−1 is assigned to a vibration of the hexanuclear cluster, in line with its absence from the IR spectra of MIL-140 A. The increased stability of UiO-66 TFA is also evidenced by the persistence of this characteristic vibration, even after 25 minutes of milling. This is in stark contrast to UiO-66 where the feature broadens and disappears before this time (Fig. S7b and c†).
Conclusions
Zr-containing MOFs, predicted to be among the most physically robust of this family of ‘soft’ porous materials, undergo rapid collapse under ball-milling. This collapse is accompanied by chemical changes (e.g., complete destruction of the clusters) beyond the simple decrease down to X-ray amorphous particle sizes, which, along with prior results for ZIFs,21 indicates that this is a general phenomenon. The unfortunate, yet intuitive result that frameworks of higher porosities30 undergo faster and more comprehensive structural collapse has important consequences for MOF mechanical processing. Addition of modulators like chloroacetic and particularly trifluoroacetic acid to UiO-66 synthesis results in striking increases in physical stability under ball-milling, despite generating more materials of almost identical porosity. In the case of the latter, TFA modulated framework, porosity is retained for at least a 10 times longer duration. A relationship between modulator pKa and material stability has been established. This is a promising concept to create ‘super-strong’ hybrid frameworks, capable of resisting the pressures or stresses involved in post-synthesis processing and shaping.Acknowledgements
T.D.B. would like to thank Trinity Hall for funding, along with Professor Anthony K. Cheetham for use of lab facilities. D.D.V. is grateful to IWT (MOF Shape), KU Leuven for support in the Metusalem grant CASAS and IAP 7/05 Functional Supramolecular Systems. I.S. and B.B. thank Research Foundation – Flanders (FWO) for Ph.D. fellowships.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974–986 CrossRef CAS PubMed.
- F. Vermoortele, M. Vandichel, B. Van de Voorde, R. Ameloot, M. Waroquier, V. Van Speybroeck and D. E. De Vos, Angew. Chem., Int. Ed., 2012, 51, 4887–4890 CrossRef CAS PubMed.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- T. D. Bennett, P. J. Saines, D. A. Keen, J. C. Tan and A. K. Cheetham, Chem.–Eur. J., 2013, 19, 7049–7055 CrossRef CAS PubMed.
- G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS PubMed.
- B. Van de Voorde, B. Bueken, J. Denayer and D. De Vos, Chem. Soc. Rev., 2014, 5766–5788 RSC.
- J. C. Tan and A. K. Cheetham, Chem. Soc. Rev., 2011, 40, 1059–1080 RSC.
- T. D. Bennett and A. K. Cheetham, Acc. Chem. Res., 2014, 47, 1555–1562 CrossRef CAS PubMed.
- D. Bazer-Bachi, L. Assié, V. Lecocq, B. Harbuzaru and V. Falk, Powder Technol., 2014, 255, 52–59 CrossRef CAS PubMed.
- G. W. Peterson, J. B. DeCoste, T. G. Glover, Y. G. Huang, H. Jasuja and K. S. Walton, Microporous Mesoporous Mater., 2013, 179, 48–53 CrossRef CAS PubMed.
- E. Proietti, F. Jaouen, M. Lefevre, N. Larouche, J. Tian, J. Herranz and J. P. Dodelet, Nat. Commun., 2011, 2, 416 CrossRef PubMed.
- T. D. Bennett, A. L. Goodwin, M. T. Dove, D. A. Keen, M. G. Tucker, E. R. Barney, A. K. Soper, E. G. Bithell, J. C. Tan and A. K. Cheetham, Phys. Rev. Lett., 2010, 104 CAS.
- T. D. Bennett, D. A. Keen, J. C. Tan, E. R. Barney, A. L. Goodwin and A. K. Cheetham, Angew. Chem., Int. Ed., 2011, 50, 3067–3071 CrossRef CAS PubMed.
- K. W. Chapman, D. F. Sava, G. J. Halder, P. J. Chupas and T. M. Nenoff, J. Am. Chem. Soc., 2011, 133, 18583–18585 CrossRef CAS PubMed.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- V. Guillerm, F. Ragon, M. Dan-Hardi, T. Devic, M. Vishnuvarthan, B. Campo, A. Vimont, G. Clet, Q. Yang, G. Maurin, G. Férey, A. Vittadini, S. Gross and C. Serre, Angew. Chem., Int. Ed., 2012, 51, 9267–9271 CrossRef CAS PubMed.
- H. Wu, T. Yildirim and W. Zhou, J. Phys. Chem. Lett., 2013, 4, 925–930 CrossRef CAS.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS PubMed.
- A. U. Ortiz, A. Boutin, A. H. Fuchs and F. X. Coudert, J. Chem. Phys., 2013, 138, 174703 CrossRef PubMed.
- J. C. Tan, B. Civalleri, C. C. Lin, L. Valenzano, R. Galvelis, P. F. Chen, T. D. Bennett, C. Mellot-Draznieks, C. M. Zicovich-Wilson and A. K. Cheetham, Phys. Rev. Lett., 2012, 108, 095502 CrossRef.
- T. D. Bennett, S. Cao, J. C. Tan, D. A. Keen, E. G. Bithell, P. J. Beldon, T. Friscic and A. K. Cheetham, J. Am. Chem. Soc., 2011, 133, 14546–14549 CrossRef CAS PubMed.
- H. K. Chae, D. Y. Siberio-Pérez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. Yaghi, Nature, 2004, 427, 523–527 CrossRef CAS PubMed.
- F. Vermoortele, B. Bueken, G. Le Bars, B. Van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2013, 135, 11465–11468 CrossRef CAS PubMed.
- P. J. Beldon, L. Fabian, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friscic, Angew. Chem., Int. Ed., 2010, 49, 9640–9643 CrossRef CAS PubMed.
- J. C. Tan, T. D. Bennett and A. K. Cheetham, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9938–9943 CrossRef CAS PubMed.
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS.
- M. J. Katz, Z. J. Brown, Y. J. Colon, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449–9451 RSC.
- L. M. Yang, E. D. Ganz, S. Svelle and M. Tilset, J. Mater. Chem. C, 2014, 2, 7111–7125 RSC.
- A. Nimmermark, L. Ohrstrom and J. Reedijk, Z. Kristallogr., 2013, 228, 311–317 CAS.
- R. L. Martin and M. Haranczyk, Chem. Sci., 2013, 4, 1781–1785 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray diffraction, thermogravimetric analysis, nitrogen physisorption and pore distribution analysis, scanning electron microscopy images, IR spectroscopy. See DOI: 10.1039/c4ta06396a |
| This journal is © The Royal Society of Chemistry 2015 |