A molecular view of the role of chirality in charge-driven polypeptide complexation†
Abstract
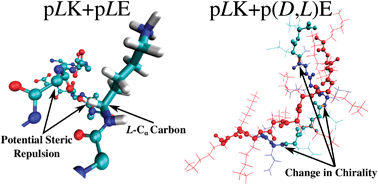
Polyelectrolyte molecules of opposite charge are known to form stable complexes in solution. Depending on the system conditions, such complexes can be solid or liquid. The latter are known as complex coacervates, and they appear as a second liquid phase in equilibrium with a polymer-dilute aqueous phase. This work considers the complexation between poly(glutamic acid) and poly(lysine), which is of particular interest because it enables examination of the role of chirality in ionic complexation, without changes to the overall chemical composition. Systematic atomic-level simulations are carried out for chains of poly(glutamic acid) and poly(lysine) with varying combinations of chirality along the backbone. Achiral chains form unstructured complexes. In contrast, homochiral chains lead to formation of stable β-sheets between molecules of opposite charge, and experiments indicate that β-sheet formation is correlated with the formation of solid precipitates. Changes in chirality along the peptide backbone are found to cause “kinks” in the β-sheets. These are energetically unfavorable and result in irregular structures that are more difficult to pack together. Taken together, these results provide new insights that may be of use for the development of simple yet strong bioinspired materials consisting of β-rich domains and amorphous regions.

 Please wait while we load your content...
Please wait while we load your content...