Open Access Article
Open Access ArticleSingly and doubly β-to-β platinum-bridged porphyrin dimers and their reductive eliminations†
Hua-Wei
Jiang
,
Takayuki
Tanaka
and
Atsuhiro
Osuka
*
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan. E-mail: osuka@kuchem.kyoto-u.ac.jp
First published on 30th July 2015
Abstract
2-Borylated porphyrins reacted with Pt(cod)Cl2 to give β-to-β platinum-bridged porphyrin dimers, which were converted to β-to-β directly linked porphyrin dimers through triphenylphosphine-mediated reductive elimination. Similar reactions of 2,18-diborylated Ni(II)–porphyrin and Zn(II)–porphyrin gave the corresponding doubly β-to-β platinum-bridged porphyrin dimers. Treatment of the doubly β-to-β platinum-bridged Ni(II)–porphyrin dimer with triphenylphosphine caused a single reductive elimination to produce a Ni(II)–porphyrin dimer possessing a β-to-β platinum bridge and a β-to-β direct C–C bond.
Introduction
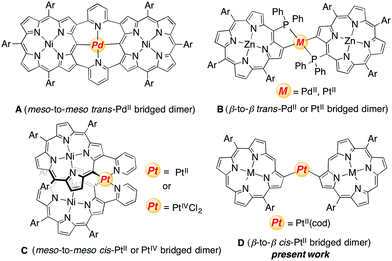
In the past decade, porphyrins bearing a metal fragment directly at their peripheries have been actively explored in light of their characteristic optical and electronic properties and catalytic reactivity.1–4 As an extension of these species, directly metal-bridged porphyrin dimers have also been developed, which display intriguing structural features and characteristic electronic interactions between the porphyrin units through the metal bridge.5 Representative examples are shown in Chart 1. The doubly 2,6-pyridylene-bridged Ni(II)–porphyrin dimer underwent smooth palladation to give dimer A, in which the two porphyrin units have meso-to-meso trans-coordination to the palladium metal, and the incorporated palladium metal increases both the electronic interaction and molecular curvature.4emeso-Diphenylphosphanyl Zn(II)–porphyrin facilitated palladation and platination at the adjacent β-position to produce dimer B, which shows β-to-β trans-coordination of the two porphyrins.2b,c Platinum-bridged dimer C formed from complexation of β-(pyrid-2-yl)-substituted Ni(II)–porphyrin with (Bu4N)2PtCl6 possesses meso-to-meso cis-coordination and shows intriguing conformational slippage upon two-electron reduction at the bridging platinum metal.4dRecently, we reported the synthesis of cyclic 2,12-porphyrinylene nanorings by following Yamago's synthetic strategy.6,7 2,12-Diborylated Ni(II)–porphyrins were transformed to platinum-bridged oligomeric porphyrin rings, which reacted with triphenylphosphine to induce reductive elimination.6 A key factor in the synthesis of such porphyrin nanorings is a preferred cis-geometry of the platinum-bridged nanoring intermediates, which causes molecular curvature, as a favorable structural feature for nanoring formation.7,8 In this paper, we examined the reactions of 2-borylporphyrins and 2,18-diborylporphyrins with Pt(cod)Cl2 to get information on the structural and electronic details of platinum-bridged porphyrin dimers. Actually, these reactions afforded singly and doubly β-to-β platinum-bridged porphyrin dimers, both of which possess cis-coordination geometries (dimer D) and thus undergo reductive elimination upon treatment with triphenylphosphine.
Results and discussion
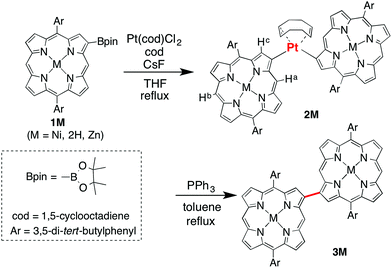
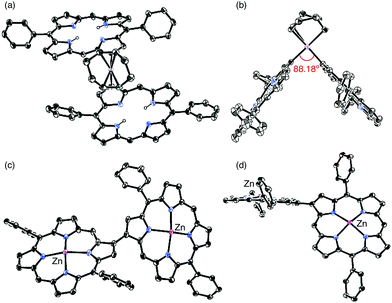
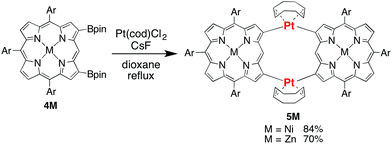
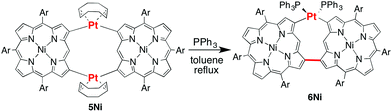
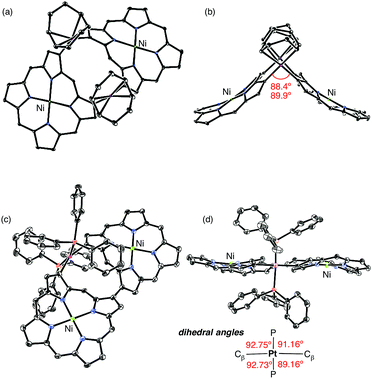
2-Borylated Ni(II) porphyrin 1Ni was treated with 0.5 equiv. Pt(cod)Cl2 in the presence of cesium fluoride and 1,5-cyclooctadiene (cod) in refluxing THF under an argon atmosphere to give β-to-β platinum-bridged porphyrin dimer 2Ni in 84% yield as a stable solid (Scheme 1).9 Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) detected the parent ion peak at m/z = 1782.73 (calcd for C104H114N858Ni2192Pt = 1782.75 [M]+). The 1H NMR spectrum of 2Ni showed singlets due to the meso-protons at 10.66 ppm (Ha) and 9.67 ppm (Hb) and a singlet due to the β-proton (Hc) at 9.12 ppm. Under the same reaction conditions, 2-borylporphyrins 1H and 1Zn were dimerised to give 2H and 2Zn in 60 and 82% yields, respectively. In these reactions, 1,5-cyclooctadiene ligand was found to be crucial, since the reactions with platinum salts with other ligands such as 1,3-bis(diphenylphosphino)propane, ethylenediamine, 2,2′-bipyridine and 2,5-norbornadiene did not give platinum-bridged dimers. The structures of 2H and 2Zn have been unambiguously confirmed by X-ray diffraction analysis (Fig. 1 and ESI†). Both dimers 2H and 2Zn display cis-arrangements of the two porphyrins at the platinum bridge with bite angles (∠Cβ–Pt–Cβ) of 88.18° and 86.33°, respectively. In addition, the two porphyrins in 2H and 2Zn take offset arrangements, as seen in their top views. The Cβ–Pt bond lengths are 2.024 and 2.036 Å in 2H, and 2.002 and 2.032 Å in 2Zn, which are longer than the Cmeso–Pt bonds in meso-to-meso cis-Pt(II) bridged Ni(II) porphyrin dimer C (1.933 Å).4c Treatment of 2Ni, 2H and 2Zn with triphenylphosphine induced reductive elimination to yield directly β–β-linked porphyrin dimers 3Ni, 3H and 3Zn in 81, 76 and 78% yields, respectively. X-ray diffraction analysis of 3Zn has revealed a β-to-β direct C–C bond with a bond distance of 1.462 Å and a dihedral angle of the two porphyrins of 57.51°. The structures of 3Ni, 3H and 3Zn are fully consistent with their spectroscopic data (ESI†).10 It is worthy to mention that meso-platinum-bridged porphyrin dimers were not obtained from meso-borylporphyrins under similar conditions, probably due to serious steric hindrance at the meso-position.In the next step, we examined the reaction of 2,18-diborylated Ni(II)–porphyrin 4Ni with an equimolar amount of Pt(cod)Cl2 in 1,4-dioxane under similar conditions, which afforded doubly β-to-β platinum-bridged porphyrin dimer 5Ni as a stable compound in 84% yield (Scheme 2). MALDI-TOF-MS showed the mass ion peak of 5Ni at m/z = 2356.95 (calcd. for C132H152N858Ni2192Pt2 = 2357.02 [M-cod]+). The 1H NMR spectrum of 5Ni exhibited a singlet at 11.86 ppm due to the meso-protons and a singlet at 8.76 ppm due to the β-protons adjacent to the platinum linkage. The doubly bridged structure of 5Ni has been confirmed by X-ray analysis, in which the two porphyrins take cis-coordination geometries with Cβ–Pt bond lengths of 2.020, 2.044, 2.021 and 2.033 Å (Fig. 2). Therefore, the two porphyrins are held in an oblique arrangement with bite angles of 88.41° and 89.89°. In addition, the two Ni(II) porphyrins take on saddle confirmations with mean-plane deviations of 0.306 Å. This is the first example of doubly and directly metal-bridged porphyrin dimer. The reaction of 4Zn with Pt(cod)Cl2 under the same conditions gave 5Zn in 70% yield. This complex was found to decompose slowly under ambient conditions but could be stored without serious deterioration under an inert atmosphere at low temperature. In contrast, the reactions of 4H with Pt(cod)Cl2 gave complicated mixtures under all conditions we tested.
Finally, the reductive elimination of 5Ni was attempted by the reaction with triphenylphosphine in refluxing toluene, which gave rise to a single platinum elimination as well as a ligand replacement of 1,5-cyclooctadiene with two triphenylphosphines to provide dimer 6Ni in 78% yield (Scheme 3). MALDI-TOF-MS showed the parent ion peak of 6Ni at m/z = 2579.21 (calcd for C160H170N858Ni2P2192Pt = 2579.14 [M]+). The 1H NMR spectrum of 6Ni exhibited a singlet at 11.79 ppm due to the meso-protons and singlets at 9.00 and 8.01 ppm due to the β-protons adjacent to the platinum bridge and the direct C–C linkage. The structure of 6Ni has been determined by X-ray analysis, and shows a β-to-β direct C–C bond with a bond distance of 1.486 Å. Owing to the structural constraint imposed by this direct β-to-β connection, the platinum bridge has now a trans coordination geometry with a large Cβ–Pt–Cβ angle of 159.04° and slightly longer Cβ–Pt bond lengths of 2.059 and 2.084 Å as compared with those of 5Ni. The two porphyrins of 6Ni show slightly larger mean-plane deviations of 0.322 and 0.329 Å. Further reductive elimination of 6Ni was attempted under stronger reaction conditions but ended in failure. This failure may be ascribed to the trans-coordination of 6Ni and severe steric hindrance in the expected doubly β-to-β connected porphyrin dimer due to the closely located meso-hydrogen atoms. The reductive elimination of 5Zn was attempted but resulted in the production of a complicated mixture. These results suggest that the central metal in the porphyrin pocket plays a vital role in these reactions.
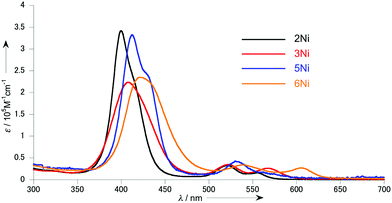
Fig. 3 shows the UV/vis absorption spectra of 2Ni, 3Ni, 5Ni and 6Ni in CH2Cl2. As compared with the sharp Soret band (λmax = 412 nm) of 5,15-diaryl Ni(II)-porphyrin,11 the Soret band of 2Ni becomes considerably broadened with a substantial blue shift to 400 nm, reflecting the exciton coupling and the influence of the platinum-bridge. The Soret band of 3Ni is observed as a much broader band at 408 nm as a consequence of the increased exciton coupling as well as the through-bond electronic interactions. The UV/vis absorption spectrum of 5Ni displays a split Soret band at 412 and 435 nm probably as a consequence of increased exciton coupling due to its fixed conformation by the double Pt bridges. Naturally, the absorption spectrum of 6Ni exhibits a much broader Soret band at 421 nm and the most red-shifted Q-band at 604 nm.
The electrochemical properties of 2Ni, 3Ni, 5Ni and 6Ni have been investigated by cyclic voltammetry and differential pulse voltammetry in benzonitrile (Table 1). The reference Ni(II) porphyrin exhibits an oxidation potential at 0.53 V and a reduction potential at −1.76 V, which leads to the estimation of an electrochemical HOMO–LUMO gap (ΔEHL) of 2.29 eV. The platinum-bridged dimers display negatively shifted oxidation and reduction potentials, at 0.36 and −1.87 V for 2Ni and 0.28 and −1.97 V for 5Ni. The DFT molecular orbital calculations have revealed that the presence of C–Pt bonds substantially raises the MO energy levels due to d–π antibonding interactions.2c,4c In other words, the platinum bridge works as an electron-donating substituent to Ni(II) porphyrin. In contrast, the direct Cβ–Cβ connection results in split frontier molecular orbitals due to interporphyrin π-orbital interaction. It is thus expected that the LUMO of 6Ni is stabilised by the electronic interaction between the two Ni(II)–porphyrins, but the HOMO is destabilised due to the electronic interaction between the two Ni(II)–porphyrins as well as the antibonding interaction with the platinum bridge. Consequently, the ΔEHL value of 6Ni is smaller than those of 2Ni and 5Ni, in line with their UV/vis absorption spectral data.
| E ox2 1/2 | E ox1 1/2 | E red1 1/2 | E red2 1/2 | ΔEHLb | |
|---|---|---|---|---|---|
| a Conditions; nBu4NPF6 electrolyte 0.1 M in PhCN, Ag/AgClO4 reference electrode, Pt working electrode, Pt wire counter electrode, scan rate 0.05 V s−1. All values were determined by differential pulse voltammetry (in V). b ΔEHL = electrochemical HOMO–LUMO gap (= Eox11/2 − Ered11/2 [eV]). c 5,15-Bis(3,5-di-tert-butylphenyl)porphyrinatonickel(II). d Irreversible peaks. | |||||
| Ni(II) porphyrinc | 0.53d | −1.76 | 2.29 | ||
| 2Ni | 0.36d | −1.87 | 2.23 | ||
| 3Ni | 0.62d | −1.69 | −1.80 | 2.31 | |
| 5Ni | 0.28d | −1.97 | 2.25 | ||
| 6Ni | 0.50 | 0.32 | −1.87 | −2.07d | 2.19 |
Conclusions
2-Borylporphyrins reacted with Pt(cod)Cl2 in the presence of cesium fluoride to produce β-to-β platinum-bridged porphyrin dimers, which were converted to β–β directly linked porphyrin dimers through triphenylphosphine-mediated reductive elimination. A similar reaction of 2,18-diborylated Ni(II)–porphyrin gave a doubly β-to-β platinum-bridged Ni(II)–porphyrin dimer, which was converted to a Ni(II)–porphyrin dimer bearing a β-to-β platinum-bridge and a β-to-β direct C–C bond via similar reductive eliminations. These platinum-bridged porphyrin dimers display characteristic electronic and optical properties. Further extension of this strategy to longer porphyrin arrays is now in progress in our laboratory.Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers (25220802 and 25620031). The authors thank Prof. Dr Hiromitsu Maeda and Dr Yuya Bando (Ritsumeikan University) for MALDI-TOF MS measurement.Notes and references
- (a) F. Atefi and D. P. Arnold, J. Porphyrins Phthalocyanines, 2008, 12, 801 CrossRef CAS; (b) B. M. J. M. Suijikerbuijk and R. J. M. Klein Gebbink, Angew. Chem., Int. Ed., 2008, 47, 7396 CrossRef PubMed; (c) H. Shinokubo and A. Osuka, Chem. Commun., 2009, 1011 RSC; (d) H. Yorimitsu and A. Osuka, Asian J. Org. Chem., 2013, 2, 356 CrossRef CAS PubMed.
- (a) D. P. Arnold, P. C. Healy, M. J. Hodgson and M. L. Williams, J. Organomet. Chem., 2000, 607, 41 CrossRef CAS; (b) Y. Matano, K. Matsumoto, Y. Nakao, H. Uno, S. Sakai and H. Imahori, J. Am. Chem. Soc., 2008, 130, 4588 CrossRef CAS PubMed; (c) Y. Matano, K. Matsumoto, H. Hayashi, Y. Nakao, T. Kumpulainen, V. Chukharev, N. Tkachenko, H. Lemmetyinen, S. Shimizu, N. Kobayashi, D. Sakamaki, A. Ito, K. Tanaka and H. Imahori, J. Am. Chem. Soc., 2012, 134, 1825 CrossRef CAS PubMed; (d) R. D. Hartnell, T. Yoneda, H. Mori, A. Osuka and D. P. Arnold, Chem.–Asian J., 2013, 8, 2670 CrossRef CAS PubMed.
- (a) K. M. Smith, K. C. Langry and O. Minnetian, J. Org. Chem., 1984, 49, 4602 CrossRef CAS; (b) K. Sugiura, A. Kato, K. Iwasaki, H. Miyasaka, M. Yamashita, S. Hino and D. P. Arnold, Chem. Commun., 2007, 2046 RSC.
- (a) S. Yamaguchi, T. Katoh, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2007, 129, 6392 CrossRef CAS PubMed; (b) J. Yamamoto, T. Shimizu, S. Yamaguchi, N. Aratani, H. Shinokubo and A. Osuka, J. Porphyrins Phthalocyanines, 2011, 15, 534 CrossRef CAS; (c) S. Yamaguchi, T. Katoh, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2008, 130, 14440 CrossRef CAS PubMed; (d) S. Yamaguchi, H. Shinokubo and A. Osuka, Inorg. Chem., 2009, 48, 795 CrossRef CAS PubMed; (e) J. Song, N. Aratani, J. H. Heo, D. Kim, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2010, 132, 11868 CrossRef CAS PubMed; (f) K. Yoshida, S. Yamaguchi, A. Osuka and H. Shinokubo, Organometallics, 2010, 29, 3997 CrossRef CAS; (g) S. Yamaguchi, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2010, 132, 9992 CrossRef CAS PubMed; (h) K. Yoshida, T. Nakashima, S. Yamaguchi, A. Osuka and H. Shinokubo, Dalton Trans., 2011, 40, 8773 RSC; (i) S. Anabuki, H. Shinokubo, N. Aratani and A. Osuka, Angew. Chem., Int. Ed., 2012, 51, 3174 CrossRef CAS PubMed; (j) K. Fujimoto, T. Yoneda, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2014, 53, 1127 CrossRef CAS PubMed.
- T. Tanaka and A. Osuka, Chem. Soc. Rev., 2015, 44, 943 RSC.
- H.-W. Jiang, T. Tanaka, H. Mori, K. H. Park, D. Kim and A. Osuka, J. Am. Chem. Soc., 2015, 137, 2219 CrossRef CAS PubMed.
- (a) S. Yamago, Y. Watanabe and T. Iwamoto, Angew. Chem., Int. Ed., 2010, 49, 757 CrossRef CAS PubMed; (b) T. Iwamoto, Y. Watanabe, Y. Sakamoto, T. Suzuki and S. Yamago, J. Am. Chem. Soc., 2011, 133, 8354 CrossRef CAS PubMed; (c) S. Yamago, E. Kayahara and T. Iwamoto, Chem. Rec., 2014, 14, 84 CrossRef CAS PubMed; (d) S. Hitosugi, T. Yamasaki and H. Isobe, J. Am. Chem. Soc., 2012, 134, 12442 CrossRef CAS PubMed; (e) T. Matsuno, S. Kamata, S. Hitosugi and H. Isobe, Chem. Sci., 2013, 4, 3179 RSC.
- (a) F. Zhang and P. Bäuerle, J. Am. Chem. Soc., 2007, 129, 3090 CrossRef CAS PubMed; (b) F. Zhang, G. Götz, H. D. F. Winkler, C. A. Schalley and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 6632 CrossRef CAS PubMed.
- H. Hata, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2005, 127, 8264 CrossRef CAS PubMed.
- Examples of β–β-linked porphyrin dimers: (a) Y. Nakamura, N. Aratani, A. Tsuda, A. Osuka, K. Furukawa and T. Kato, J. Porphyrins Phthalocyanines, 2003, 7, 264 CrossRef CAS; (b) H. Uno, Y. Kitawaki and N. Ono, Chem. Commun., 2002, 116 RSC; (c) Y. Deng, C. K. Chang and D. G. Nocera, Angew. Chem., Int. Ed., 2000, 39, 1066 CrossRef CAS.
- A. Tsuda, A. Nakano, H. Furuta, H. Yamochi and A. Osuka, Angew. Chem., Int. Ed., 2000, 39, 558 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and computational details, as well as X-ray crystallographic data for 2H, 2Zn, 3Zn, 5Ni and 6Ni are available. CCDC 1406329–1406332 and 1406343. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc02553b |
| This journal is © The Royal Society of Chemistry 2015 |