Open Access Article
Open Access ArticleA hexanuclear gold carbonyl cluster†‡
Sonia
Martínez-Salvador
a,
Larry R.
Falvello
 b,
Antonio
Martín
a and
Babil
Menjón
b,
Antonio
Martín
a and
Babil
Menjón
 *a
*a
aInstituto de Síntesis Química y Catálisis Homogénea (iSQCH), CSIC – Universidad de Zaragoza, C/Pedro Cerbuna 12, E-50009 Zaragoza, Spain. E-mail: menjon@unizar.es
bInstituto de Ciencia de Materiales de Aragón (ICMA), CSIC – Universidad de Zaragoza, C/Pedro Cerbuna 12, E-50009 Zaragoza, Spain
First published on 15th June 2015
Abstract
The hexanuclear gold carbonyl cluster [PPh4]2[Au6(CF3)6Br2(CO)2] (4) has been obtained by spontaneous self-assembly of the following independent units: CF3AuCO (1) and [PPh4][Br(AuCF3)2] (3). The cyclo-Au6 aggregate 4, in which the components are held together by unassisted, fairly strong aurophilic interactions (Au⋯Au ∼310 pm), exhibits a cyclohexane-like arrangement with chair conformation. These aurophilic interactions also result in significant ν(CO) lowering: from 2194 cm−1 in the separate component 1 to 2171 cm−1 in the mixed aggregate 4. Procedures to prepare the single-bridged dinuclear component 3 as well as the mononuclear derivative [PPh4][CF3AuBr] (2) are also reported.
Introduction
There is much current interest in the study of gold carbonyls, mainly because of both the intriguing nature of the [Au]–CO bond and their involvement in gold-catalyzed transformations of CO, including its low-temperature oxidation by molecular oxygen as well as water-gas shift reaction.3 Direct unambiguous information on the active species in the latter processes is still lacking.4 IR spectroscopy is a powerful tool helping to assign the oxidation state of the active sites on the catalyst surface as well as to evaluate the influence of the chemical environment.4,5 Well-established gold carbonyls for appropriate comparison are therefore valuable species, although they still remain scarce.6 Aside from the following dinuclear derivatives: Cl3Au(μ-Cl)AuCO,7 (tBuFO)3Al(μ-Cl)AuCO and [Cl(AuCO)2][Al(OtBuF)4],8 the handful of currently isolated gold carbonyls are all mononuclear, either neutral XAuCO (X = Cl,9 CF3,10 OSO2F,11 HB(pz*)312) or cationic LAuCO+ species (L = CO,11,13 SIDipp,14,15 IDipp,15 PMes3,14,16 DPCb17). Additionally, some oligomers of unknown nuclearity have been suggested to form in frozen concentrated solutions of ClAuCO in CH2Cl2.18To the best of our knowledge, discrete polynuclear gold carbonyl compounds featuring both the Au–CO and Au⋯Au interactions are still lacking. Such species, if experimentally available, might illustrate two key interactions occurring on the catalyst surface: (1) CO coordination to an exposed gold center, and (2) cohesive bonding interaction of this active site with the bulk catalyst support. Here we report on a discrete hexanuclear gold carbonyl cluster formed by spontaneous self-assembly of independent Au1 and Au2 units, which are held together by unassisted aurophilic interactions alone.§19–21 It is further shown that such aurophilic interactions have a distinct effect on the vibrational properties of the highly sensitive [Au]–CO unit, which can therefore act as a fine probe of the molecular environment.
Results and discussion
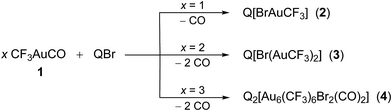
The gold carbonyl compound CF3AuCO (1) has already proven to be a useful synthon of the ‘AuCF3’ fragment toward neutral ligands.10 We have found now that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
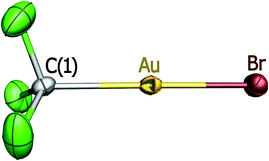
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of compound 1 with [PPh4]Br proceeds in a similar way giving rise to CO replacement (Scheme 1) with formation of the mononuclear anionic derivative [PPh4][CF3AuBr] (2). This air- and moisture-stable compound shows a typical linear arrangement (Fig. 1).
1 reaction of compound 1 with [PPh4]Br proceeds in a similar way giving rise to CO replacement (Scheme 1) with formation of the mononuclear anionic derivative [PPh4][CF3AuBr] (2). This air- and moisture-stable compound shows a typical linear arrangement (Fig. 1).
 | ||
| Scheme 1 Synthetic procedures affording the trifluoromethyl-gold derivatives 2–4. In all cases Q+ = [PPh4]+. | ||
The coordination of bromide results in significant lengthening of the Au–C distance (211.9(4) pm) with respect to that observed in both the neutral parent species 1 (204.7(14) pm)10 and the homoleptic anionic derivative [NBu4][Au(CF3)2] (203.3(2) pm).10a The Au–Br distance (240.5(1) pm) is, in turn, comparable to that found in the homoleptic complex halide [NBu4][AuBr2] (237.6(3) pm).22 In the crystal lattice of compound 2, the anions are separated by the cations resulting in large intermetallic separations (>0.7 nm).
The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
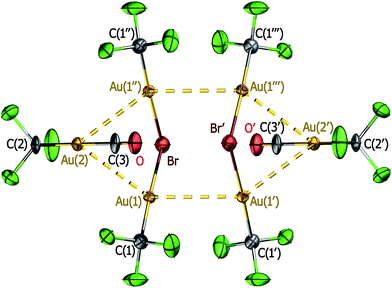
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of compound 1 with [PPh4]Br gives rise (Scheme 1) to the dinuclear species [PPh4][Br(AuCF3)2] (3) containing single-bridging bromide. In the crystal, the dinuclear anions are self-assembled in pairs (Fig. 2) showing significant intermolecular aurophilic interactions (av. Au⋯Au′ ∼326 pm), which apparently counteract Coulomb repulsion between the constituent anions in the dimer. The intramolecular intermetallic separation within each dinuclear [Br(AuCF3)2]− anion is, in turn, too long to denote any bonding interaction: av. Au⋯Au′ ∼368 pm. Similar structural patterns have been found in certain halonium [X(AuPR3)2]+ salts.23 Recent theoretical studies on the model halonium monocations [X(AuPH3)2]+ (X = F, Cl, Br, I) at the Xα level conclude that they should also undergo self-assembly against Coulomb repulsion to form tetranuclear [{X(AuPH3)2}2]2+ clusters, whereby the intermolecular interaction energy for each Au⋯Au pair was estimated to be 85.0 kJ mol−1 on average.24 It has also been suggested that such type of associations might be counterion-mediated.25 With regard to this possibility, it is interesting to note that no pairwise association was, in fact, observed in several other [X(AuL)2]+ salts,26 including the recently reported chloronium gold carbonyl derivative [Cl(AuCO)2][Al(OtBuF)4].8
1 reaction of compound 1 with [PPh4]Br gives rise (Scheme 1) to the dinuclear species [PPh4][Br(AuCF3)2] (3) containing single-bridging bromide. In the crystal, the dinuclear anions are self-assembled in pairs (Fig. 2) showing significant intermolecular aurophilic interactions (av. Au⋯Au′ ∼326 pm), which apparently counteract Coulomb repulsion between the constituent anions in the dimer. The intramolecular intermetallic separation within each dinuclear [Br(AuCF3)2]− anion is, in turn, too long to denote any bonding interaction: av. Au⋯Au′ ∼368 pm. Similar structural patterns have been found in certain halonium [X(AuPR3)2]+ salts.23 Recent theoretical studies on the model halonium monocations [X(AuPH3)2]+ (X = F, Cl, Br, I) at the Xα level conclude that they should also undergo self-assembly against Coulomb repulsion to form tetranuclear [{X(AuPH3)2}2]2+ clusters, whereby the intermolecular interaction energy for each Au⋯Au pair was estimated to be 85.0 kJ mol−1 on average.24 It has also been suggested that such type of associations might be counterion-mediated.25 With regard to this possibility, it is interesting to note that no pairwise association was, in fact, observed in several other [X(AuL)2]+ salts,26 including the recently reported chloronium gold carbonyl derivative [Cl(AuCO)2][Al(OtBuF)4].8
The av. Au–C distance in compound 3 (194(2) pm) is significantly shorter than in the mononuclear derivative 2 (211.9(5) pm), whereas the av. Au–Br distance in the single-bridging system of compound 3 (244.2(3) pm) is just slightly longer than that found in the terminal Au–Br bond of 2 (240.5(1) pm). The geometry of the Au–Br–Au unit in the anionic compound 3 is very similar to that found in the cationic derivative [Br(AuPPh3)2][SbF6], which is also associated in pairs in the solid state.23 The relationship between these cationic [X(AuPR3)2]+ and anionic [Br(AuCF3)2]− species illustrates the already pointed functional similarity between the corresponding cationic ‘AuPR3+’ and neutral ‘AuCF3’ fragments, regardless of their different global charge.10a
We considered whether the strong electrophilic ‘AuCF3’ neutral fragment would enable to build a trinuclear [(μ3-Br)(AuCF3)3]− derivative. To this aim, compound 1 was reacted with [PPh4]Br in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. However, instead of the targeted trinuclear species, a hexanuclear compound with formula [PPh4]2[Au6(CF3)6Br2(CO)2] (4) was obtained (Scheme 1). The reaction outcome makes it clear that the residual nucleophilicity of bridging bromide, μ2-Br, in component 3 is not sufficient to expel the extremely labile CO ligand in component 1. Attempts to force the release of CO resulted in extensive decomposition.
1 ratio. However, instead of the targeted trinuclear species, a hexanuclear compound with formula [PPh4]2[Au6(CF3)6Br2(CO)2] (4) was obtained (Scheme 1). The reaction outcome makes it clear that the residual nucleophilicity of bridging bromide, μ2-Br, in component 3 is not sufficient to expel the extremely labile CO ligand in component 1. Attempts to force the release of CO resulted in extensive decomposition.
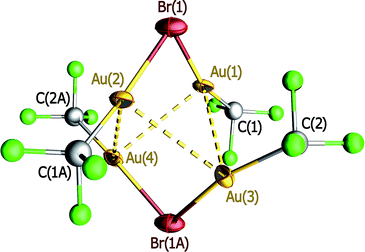
The precise nature of compound 4 was established by X-ray diffraction methods. The crystal lattice is formed by large cations and anions with little polarizing effect on each other and showing no sign of significant extra covalent interactions between them.¶27–32 The anion (Fig. 3) is, in fact, an aggregate of the following components: the anion of compound 3 and the neutral precursor species 1. The components are held together solely by aurophilic interactions of similar strength: Au⋯Au ∼310 pm. The six gold(I) atoms exhibit a cyclohexane-like arrangement with chair conformation, cyclo-Au6, of which just a single precedent is known.33 Upon aggregation, the Au–Br–Au angle in component 3 closes from 97.6(1) to 90.28(3)°, probably to favor the aurophilic interaction with component 1. All these interactions are broken in solution, since the 19F NMR signals of aggregate 4 dissolved in CH2Cl2 correspond to the separate components 1 + 3 in the appropriate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (see Experimental in ESI‡). Solid samples of 4 at room temperature show no sign of luminescent behavior by UV irradiation.
1 ratio (see Experimental in ESI‡). Solid samples of 4 at room temperature show no sign of luminescent behavior by UV irradiation.
The stereochemistry of hexanuclear gold clusters is largely dominated by a planar arrangement of the six gold centers in the metal core. For instance, the most simple neutral Au6 and charged (Au6)± bare clusters34 as well as different [Au6(CO)n]− adsorbates (n = 1–3)35 and even Au6Hn (n = 1–6) binary hydrides36 all have been assigned triangular planar structures following theoretical calculations. Cyclic planar arrangements of the gold centers were experimentally found in the homoleptic neutral compounds [Au6(SMes′)6],37 [Au6(PtBu2)6] and [Au6(PCy2)6],38 as well as in the heteroleptic [Au6Cl4(dpmppm)2]2+ cation containing double-bridging tetraphosphine ligands.39 The precise arrangement of gold centers in hexanuclear gold clusters, however, can be imposed by the presence of heteroatoms and/or additional centers that may exert a template effect. Thus, whereas the six gold(I) atoms in the heterometallic [Ag(AuMes′)6]+ cation build a planar hexagonal ring around the central Ag+ ion,40 they were found to nucleate in an octahedral fashion around the hyperaurated C atom in the [C(AuPPh3)6]2+ cation.41 Even the nature of the ancillary ligands is known to play a key role on the stereochemistry of hexanuclear gold clusters. As an example, a standard planar metal core was experimentally found in [Au6(S2CR)6] (R = o-tolyl),42 whereas a unique chair conformation was observed in [Au6(S2CNR2)6] (R = aza-15-crown-5).33 The latter arrangement was considered exceptional and it was attributed to the steric effect of the bulky R substituent. Moreover, it has also been noted that “sometimes, the geometry is not imposed by the nature of the bridging ligand(s) but by aurophilicity”.20e Compound 4 is a nice example of this particular behavior whereby, in the absence of significant steric constraints, the structure of the metal core is driven by aurophilic interactions. Recently, the effect of both aurophilic interactions and the nature of ancillary ligands on the self-assembly of complex molecular systems, as well as on the rich structural diversity found in the family of AuI thio-derivatives, has been studied in detail.43
Compound 4 provides a unique opportunity to compare the structural and spectroscopic properties of a gold carbonyl molecule, namely CF3AuCO, in two different and well defined chemical environments: (a) in isolated pure form (1), and (b) adsorbed onto a gold framework (4). None of the structural parameters of component 1 are substantially modified in aggregate 4. However, the ν(CO) vibration undergoes a significant frequency decrease upon aggregation. In the separate component 1, each CF3AuCO molecule is surrounded by three identical neighbors with trigonal symmetry, resulting in an extended three-dimensional network of weak aurophilic interactions (Au⋯Au 345.9(1) pm).10 In solid samples of component 1, ν(CO) = 2194 cm−1, whereas in the cyclo-Au6 aggregate 4 featuring substantially stronger aurophilic interactions (Au⋯Au ∼310 pm) the value drops to ν(CO) = 2171 cm−1. Recent theoretical calculations on CF3AuCO and related systems predicted that aurophilic interactions might have an impact on the vibrational properties of the [Au]–CO unit.14,44 We now provide experimental support for this prediction, as it is found that aurophilic interactions do have a distinct effect on the ν(CO) frequency. The experimentally observed trend is, however, opposite to the theoretical predictions: the stronger the interaction, the lower the ν(CO) frequency.
It is well known that [Au(L)]+ and ‘AuX’ fragments—both having single vacant coordination sites—are isolobal with the simplest Lewis acid, proton H+.45 This isolobal relationship enables to establish a formal comparison between our CF3AuCO molecule and the formyl cation HCO+. The ν(CO) frequency of this protonated carbonyl is known to lower from 2184 cm−1 in the gas phase46 to 2110 cm−1 in the condensed phase, namely in superacidic HF(l)/SbF5 medium.47 Such a large decrease was attributed to interaction of the HCO+ cation with SbF5 or anionic species derived thereof. This frequency lowering by interaction of HCO+ with extremely weak nucleophiles|| is in keeping with our experimental finding with the isolobal CF3AuCO molecule, where the ν(CO) frequency drops from 2194 cm−1 in the separate component 1 to 2171 cm−1 in the cyclo-Au6 aggregate 4 owing to fairly strong aurophilic interactions.
Conclusions
Compound 4, formed by spontaneous self-assembly of smaller pre-existing units (Scheme 1), is the first polynuclear gold carbonyl derivative to have been structurally characterized (Fig. 3). A cyclohexane-like arrangement in chair conformation was found for the cyclo-Au6 metal core even in the absence of significant conformational locks. In compound 4, the neutral gold carbonyl molecule CF3AuCO appears to be adsorbed onto a gold framework without the need of any kind of assistance. Since aurophilic interactions are the only cohesive forces holding together this supramolecular aggregate, it can be taken as a model for the local environment of gold carbonyl species adsorbed onto gold layers (Scheme 2). Compound 4 is a unique species because of its high nuclearity and also because it enables to check the influence of the strength of aurophilic interactions on the properties of the highly sensitive [Au]–CO unit. We have now experimentally established that aurophilic interactions, although weak in nature, are able to produce significant changes in the ν(CO) value of gold carbonyls, as had been theoretically predicted.14,44 This result should be taken into account when considering the properties of [Au]–CO units on the surface of supported gold catalysts.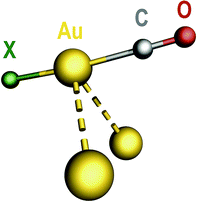 | ||
| Scheme 2 Model for local coordination environment of a [Au]–CO unit of an exposed gold site on a gold surface (X stands for any monodentate substituent). | ||
Abbreviations
| av. | Average |
| Cy | Cyclohexyl |
| DPCb | 1,2-Bis(diaminophosphino)-1,2-dicarba-closo-dodecaborane |
| dpmppm | meso-Bis[(diphenylphosphinomethyl)phenylphosphino]methane |
| IDipp | 1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene |
| Mes | 2,4,6-Trimethylphenyl (mesityl) |
| Mes′ | 2,4,6-Tris(isopropyl)phenyl |
| pz* | 3,5-Bis(trifluoromethyl)pyrazolyl |
| SIDipp | 1,3-Bis(2,6-diisopropylphenyl)imidazolin-2-ylidene |
| tBuF | Perfluoro-tert-butyl |
Acknowledgements
This work was supported by the Spanish MINECO/FEDER (Project CTQ2012-35251), and the Gobierno de Aragón (Grupo Consolidado E21: Química Inorgánica y de los Compuestos Organometálicos).Notes and references
- Encyclopedia of Inorganic Chemistry, ed. R. B. King, John Wiley & Sons, Chichester, 2nd edn, 2005, p. 926, DOI:10.1002/0470862106.id150.
- F. A. Cotton, in Metal Clusters in Chemistry, ed. P. Braunstein, L. A. Oro and P. R. Raithby, Wiley-VCH, Weinheim, 1999, vol. 1, ch. 1, pp. 2–7 and ref. given therein Search PubMed.
- T. Takei, T. Akita, I. Nakamura, T. Fujitani, M. Okumura, K. Okazaki, J. Huang, T. Ishida and M. Haruta, Adv. Catal., 2012, 55, 1 Search PubMed; M. Haruta and M. Daté, Appl. Catal., A, 2001, 222, 427 CrossRef CAS; G. C. Bond and D. T. Thompson, Gold Bull., 2000, 33, 41 CrossRef; A. I. Kozlov, A. P. Kozlova, H. Liu and Y. Iwasawa, Appl. Catal., A, 1999, 182, 9 CrossRef; G. C. Bond and D. T. Thompson, Catal. Rev. Sci. Eng., 1999, 41, 319 Search PubMed; M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, J. Catal., 1989, 115, 301 CrossRef; M. Haruta, T. Kobayashi, H. Sano and N. Yamada, Chem. Lett., 1987, 405 CrossRef.
- R. Meyer, C. Lemire, S. K. Shaikhutdinov and H.-J. Freund, Gold Bull., 2004, 37, 72 CrossRef CAS.
- F. Zaera, Chem. Soc. Rev., 2014, 43, 7624 RSC; F. Zaera, ChemCatChem, 2012, 4, 1525 CrossRef CAS; M. Mihaylov, H. Knözinger, K. Hadjiivanov and B. C. Gates, Chem. Ing. Tech., 2007, 79, 795 CrossRef.
- D. B. dell'Amico, L. Labella, F. Marchetti and S. Samaritani, Coord. Chem. Rev., 2010, 254, 635 CrossRef CAS; J. J. Rack and S. H. Strauss, Catal. Today, 1997, 36, 99 CrossRef; D. B. dell'Amico and F. Calderazzo, Gold Bull., 1997, 30, 21 CrossRef.
- D. B. dell'Amico, F. Calderazzo, P. Robino and A. Segre, J. Chem. Soc., Dalton Trans., 1991, 3017 RSC.
- J. Schaefer, A. Kraft, S. Reininger, G. Santiso-Quiñones, D. Himmel, N. Trapp, U. Gellrich, B. Breit and I. Krossing, Chem.–Eur. J., 2013, 19, 12468 CrossRef CAS PubMed.
- D. B. dell'Amico, F. Calderazzo, H. H. Murray and J. P. Fackler, Inorg. Synth., 1986, 24, 236 CrossRef CAS; P. G. Jones, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1982, 37, 823 Search PubMed; D. B. dell'Amico, F. Calderazzo and G. dell'Amico, Gazz. Chim. Ital., 1977, 107, 101 Search PubMed; D. B. dell'Amico, F. Calderazzo and F. Marchetti, J. Chem. Soc., Dalton Trans., 1976, 1829 RSC; D. B. dell'Amico and F. Calderazzo, Gazz. Chim. Ital., 1973, 103, 1099 Search PubMed; M. S. Kharasch and H. S. Isbell, J. Am. Chem. Soc., 1930, 52, 2919 CrossRef; W. Manchot and H. Gall, Ber. Dtsch. Chem. Ges. B, 1925, 58, 2175 CrossRef.
- (a) S. Martínez-Salvador, L. R. Falvello, A. Martín and B. Menjón, Chem.–Eur. J., 2013, 19, 14540 CrossRef PubMed; (b) S. Martínez-Salvador, J. Forniés, A. Martín and B. Menjón, Angew. Chem., Int. Ed., 2011, 50, 6571 CrossRef PubMed.
- H. Willner and F. Aubke, Inorg. Chem., 1990, 29, 2195 CrossRef CAS.
- H. V. R. Dias and W. Jin, Inorg. Chem., 1996, 35, 3687 CrossRef CAS.
- R. Küster and K. Seppelt, Z. Anorg. Allg. Chem., 2000, 626, 236 CrossRef CAS; C. Wang, S. C. Siu, G. Hwang, C. Bach, B. Bley, M. Bodenbinder, H. Willner and F. Aubke, Can. J. Chem., 1996, 74, 1952 CrossRef; H. Willner, J. Schaebs, G. Hwang, F. Mistry, R. Jones, J. Trotter and F. Aubke, J. Am. Chem. Soc., 1992, 114, 8972 CrossRef; M. Adelhelm, W. Bacher, E. G. Höhn and E. Jacob, Chem. Ber., 1991, 124, 1559 CrossRef.
- M. A. Celik, C. Dash, V. A. K. Adiraju, A. Das, M. Yousufuddin, G. Frenking and H. V. R. Dias, Inorg. Chem., 2013, 52, 729 CrossRef CAS PubMed.
- C. Dash, P. Kroll, M. Yousufuddin and H. V. R. Dias, Chem. Commun., 2011, 47, 4478 RSC.
- H. V. R. Dias, C. Dash, M. Yousufuddin, M. A. Celik and G. Frenking, Inorg. Chem., 2011, 50, 4253 CrossRef CAS PubMed.
- M. Joost, L. Estévez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune and D. Bourissou, Angew. Chem., Int. Ed., 2014, 53, 14512 CrossRef CAS PubMed.
- O. Elbjeirami, S. Yockel, C. F. Campana, A. K. Wilson and M. A. Omary, Organometallics, 2007, 26, 2550 CrossRef CAS.
- H. G. Raubenheimer and H. Schmidbaur, J. Chem. Educ., 2014, 91, 2024 CrossRef CAS; H. Schmidbaur, Gold Bull., 2000, 33, 3 CrossRef; H. Schmidbaur, Gold Bull., 1990, 23, 11 CrossRef.
- (a) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC; (b) R. J. Puddephatt, Chem. Soc. Rev., 2008, 37, 2012 RSC; (c) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931 RSC; (d) M. J. Katz, K. Sakaib and D. B. Leznoff, Chem. Soc. Rev., 2008, 37, 1884 RSC; (e) J. Vicente, M. T. Chicote, I. Saura-Llamas, M. C. Lagunas, M. C. Ramírez de Arellano, P. González-Herrero, M. D. Abrisqueta and R. Guerrero, in Metal Clusters in Chemistry, ed. P. Braunstein, L. A. Oro and P. R. Raithby, Weinheim, 1999, vol. 1, ch. 1.26, pp. 493–508 Search PubMed; (f) H. Schmidbaur, Chem. Soc. Rev., 1995, 24, 391 RSC.
- E. R. T. Tiekink and J.-G. Kang, Coord. Chem. Rev., 2009, 253, 1627 CrossRef CAS; V. W.-W. Yam and E. C.-C. Cheng, Chem. Soc. Rev., 2008, 37, 1806 RSC; V. W.-W. Yam and E. C.-C. Cheng, Top. Curr. Chem., 2007, 281, 269 CrossRef.
- P. Braunstein, A. Müller and H. Bögge, Inorg. Chem., 1986, 25, 2104 CrossRef CAS.
- H. Schmidbaur, A. Hamel, N. W. Mitzel, A. Schier and S. Nogai, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4916 CrossRef CAS PubMed; A. Hamel, N. W. Mitzel and H. Schmidbaur, J. Am. Chem. Soc., 2001, 123, 5106 CrossRef PubMed.
- H. Fang, X.-G. Zhang and S.-G. Wang, Phys. Chem. Chem. Phys., 2009, 11, 5796 RSC.
- M. A. Carvajal, S. Alvarez and J. J. Novoa, Theor. Chem. Acc., 2006, 116, 472 CrossRef CAS.
- Y. Zhu, C. S. Day, L. Zhang, K. J. Hauser and A. C. Jones, Chem.–Eur. J., 2013, 19, 12264 CrossRef CAS PubMed; A. Homs, I. Escofet and A. M. Echavarren, Org. Lett., 2013, 15, 5782 CrossRef PubMed; A. Bayler, A. Bauer and H. Schmidbaur, Chem. Ber., 1997, 130, 115 CrossRef; P. G. Jones, G. M. Sheldrick, R. Usón and A. Laguna, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 1486 CrossRef; R. Usón, A. Laguna and M. V. Castrillo, Synth. React. Inorg. Met.-Org. Chem., 1979, 9, 317 CrossRef.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19 RSC.
- J. J. McKinnon, D. Jayatilaka and M. A. Spackman, Chem. Commun., 2007, 3814 RSC; M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378 RSC.
- T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 48 CrossRef CAS; G. A. Jeffrey, An Introduction to Hydrogen Bonding, Oxford University Press, Oxford, 1997 Search PubMed.
- H. Willner and F. Aubke, Organometallics, 2003, 22, 3612 CrossRef CAS; H. Willner and F. Aubke, Chem.–Eur. J., 2003, 9, 1668 CrossRef PubMed; H. Willner and F. Aubke, in Inorganic Chemistry Highlights, ed. G. Meyer, D. Naumann and L. Wesemann, Wiley-VCH, Weinheim, 2002, ch. 11, pp. 195–212 CrossRef; H. Willner and F. Aubke, Angew. Chem., Int. Ed. Engl., 1997, 36, 2402 CrossRef.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
- B. von Ahsen, M. Berkei, G. Henkel, H. Willner and F. Aubke, J. Am. Chem. Soc., 2002, 124, 8371 CrossRef CAS PubMed.
- J. Arias, M. Bardají and P. Espinet, Inorg. Chem., 2008, 47, 1597 CrossRef CAS PubMed.
- P. Pyykkö, Annu. Rev. Phys. Chem., 2012, 63, 45 CrossRef PubMed.
- H.-J. Zhai, B. Kiran, B. Dai, J. Li and L.-S. Wang, J. Am. Chem. Soc., 2005, 127, 12098 CrossRef CAS PubMed.
- S. Baishya and R. C. Deka, Comput. Theor. Chem., 2012, 1002, 1 CrossRef CAS.
- D. J. LeBlanc and C. J. L. Lock, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1997, 53, 1765 Search PubMed; I. Schröter and J. Strähle, Chem. Ber., 1991, 124, 2161 CrossRef.
- D. M. Stefanescu, H. F. Yuen, D. S. Glueck, J. A. Golen and A. L. Rheingold, Angew. Chem., Int. Ed., 2003, 42, 1046 CrossRef CAS PubMed.
- Y. Takemura, H. Takenaka, T. Nakajima and T. Tanase, Angew. Chem., Int. Ed., 2009, 48, 2157 CrossRef CAS PubMed.
- E. Cerrada, M. Contel, A. D. Valencia, M. Laguna, T. Gelbrich and M. B. Hursthouse, Angew. Chem., Int. Ed., 2000, 39, 2353 CrossRef CAS.
- F. Scherbaum, A. Grohmann, B. Huber, C. Krüger and H. Schmidbaur, Angew. Chem., Int. Ed. Engl., 1988, 27, 1544 CrossRef.
- J. A. Schuerman, F. R. Fronczek and J. Selbin, J. Am. Chem. Soc., 1986, 108, 336 CrossRef CAS.
- M.-R. Azani, O. Castillo, M. L. Gallego, T. Parella, G. Aullón, O. Crespo, A. Laguna, S. Alvarez, R. Mas-Ballesté and F. Zamora, Chem.–Eur. J., 2012, 18, 9965 CrossRef CAS PubMed.
- Z.-F. Li, X.-P. Yang, L. Hui-Xue and Z. Guo, Organometallics, 2014, 33, 5101 CrossRef CAS; O. Elbjeirami, S. Yockel, C. F. Campana, A. K. Wilson and M. A. Omary, Organometallics, 2007, 26, 2550 CrossRef.
- H. G. Raubenheimer and H. Schmidbaur, Organometallics, 2012, 31, 2507 CrossRef CAS.
- P. B. Davies, P. A. Hamilton and W. J. Rothwell, J. Chem. Phys., 1984, 81, 1598 CrossRef CAS; S. C. Foster, A. R. W. McKellar and T. J. Sears, J. Chem. Phys., 1984, 81, 578 CrossRef.
- P. J. F. de Rege, J. A. Gladysz and I. T. Horváth, Science, 1997, 276, 776 CrossRef CAS PubMed.
- R. Friedemann and K. Seppelt, Eur. J. Inorg. Chem., 2013, 1197 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. Dr Pablo Espinet on the occasion of his 65th birthday. Abbreviations are defined prior to the Acknowledgements section. As defined in ref. 1, “clusters are molecular units which may contain small or large numbers of similar atoms where there are several short internuclear distances between atom pairs.” This definition is fully compatible with that originally given by F. A. Cotton (ref. 2). |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, comments on the X-ray structure determinations and 3D Hirschfeld surfaces for the ions constituting the crystal of 4. CCDC 1049919–1049921. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc01578b |
| § Aurophilic interactions are among the most important secondary interactions currently known in chemistry.19 Although usually weak in nature, they are instrumental in shaping a most stunning variety of clusters, polynuclear compounds and supramolecular frameworks.20 Aurophilic interactions also have a marked influence on the electronic properties of the chemical entity, thus enabling the fine-tuning of the energy levels and hence of the optical properties thereby involved.21 The effect on other fundamental spectroscopic properties, however, is by far less known. |
| ¶ Three-dimensional Hirschfeld surfaces (HSs) have been calculated for each ion constituting the crystal of 4 (Fig. S3 and S4 in ESI‡).27 These HSs are unique to each constituent unit and provide a convenient way for comparison of intermolecular contacts relative to van der Waals radii through a simple red–white–blue colour scheme.28 It becomes clear that the ions constituting the crystal lattice of 4 are virtually non-interacting. Just a few small, red spots of poor intensity are to be located in the corresponding HS, which have been specifically analyzed and considered of little (if any) significance as justified in what follows. In particular, the interatomic distances associated with the few, seeming H-bonds are at the long end of the commonly admitted range,29 and the angles between the atoms involved deviate significantly from linearity (<135°). These geometric parameters jointly indicate extremely poor interactions. The possibility of significant F⋯CO interactions of both intra- and inter-molecular character was also considered. Such interactions are known to be favored in some salts of superelectrophilic metal carbonyl cations [M(CO)x]q− with fluorinated [SbF6·nSbF5]− anions—n, q and x being integers—and have been thoroughly studied.30 F⋯CO contacts at >300 pm, as found in compound 4, are well within the 10% safety margin of the sum of the corresponding van der Waals radii, rvdW(F) + rvdW(C) = 317 pm,31 and have been previously considered as of just marginal importance.30,32 |
| || Anions such as [SbF6]− or [Sb2F11]− typically behave as extremely poor nucleophiles. Nevertheless, they are known to interact with sufficiently strong electrophilic centers, as for instance, in (PF3)2Pt(SbF6)2 and (P⁁P)Pt(Sb2F11), where P⁁P = (CF3)2PCH2CH2P(CF3)2.48 This is most probably the case of the HCO+ cation in HF(l)/SbF5 medium.47 |
| This journal is © The Royal Society of Chemistry 2015 |