Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bypassing the lack of reactivity of endo-substituted norbornenes with the catalytic rectification–insertion mechanism†
Basile
Commarieu
and
Jerome P.
Claverie
*
Quebec Center for Functional Materials, UQAM, Dept of Chemistry, Succ Centre Ville CP8888, Montreal H3C3P8, Qc, Canada. E-mail: claverie.jerome@uqam.ca
First published on 24th December 2014
Abstract
The catalytic 1,2-insertion polymerization of polar norbornenes (NBEs) leads to the formation of functional rigid macromolecules with exceptional thermal, optical and mechanical properties. However, this remarkable reaction is plagued by the low reactivity of the polar monomers, and most notably of those bearing a functional group in endo position. We have examined the polymerization mechanism of NBEs bearing one or two CO2Me groups either in exo or endo position catalyzed by the so-called naked allyl Pd+ SbF6− catalyst (1). Although endo dimethyl ester of 5-norbornene-2,3-dicarboxylic acid (NBE(CO2Me)2) is polymerized by 1, two endo units are never inserted consecutively along the polymer chain. Indeed, 1 is a tandem catalyst which not only catalyzes the insertion of the monomer but also the isomerization of endo and exo isomers. Thus, the polymerization of endo monomers proceeds via a novel mechanism, coined rectification–insertion mechanism, whereby half of the endo monomers are rectified into exo ones prior insertion, leading to the formation of an alternating endo–exo copolymer using an endo only feedstock. With this mechanism, the lack of reactivity of endo norbornenes is bypassed, and the polymerization of predominantly endo polar NBEs bearing a variety of functionalities such as esters, imides, acids, aldehydes, alcohols, anhydrides, or alkyl bromides proceeds with catalyst loadings as low as 0.002 mol%.
Introduction
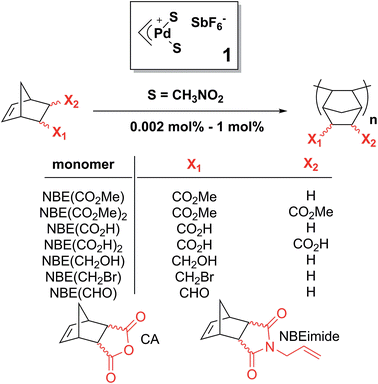
Functional macromolecules are essential components for the formation of complex nanostructures with defined shape and functionality with applications in optical and electronic materials, catalysis, recognition/separation technologies and drug delivery.1 Among those, polynorbornene (PNBE) prepared by 1,2-insertion catalytic polymerization is particularly interesting as it has excellent thermal and chemical stability (degradation above 400 °C), high Tg (>300 °C), low dielectric constant and low birefringence.2,3 Furthermore, when disyndiotactic, this rigid polymer adopts a distinctive tubular helical molecular crystalline structure.4 Polar norbornenes (NBEs) can be prepared from straightforward Diels–Alder reactions between cyclopentadiene and simple dienophiles. Thus, one envisions that the polymerization of polar NBEs could become a remarkably efficient and green route to prepare functional polymers which could eventually rival in practicality with controlled radical polymerization,5–7 group-transfer polymerization8,9 or metathesis-based polymerizations.10–14 However, despite the existence of numerous NBE polymerization catalysts based on either early transition metals such as Zr or V or late transition metals such as Pd or Ni,15–18 the polymerization of polar NBEs is still plagued by several ineluctable issues. First, and probably most importantly, NBEs bearing endo polar substituents are known to deactivate the catalyst,15–17 so that exo and endo isomers must be separated using time-consuming techniques in order to achieve high yields. Furthermore, Lewis acids such as alkyl aluminums, methylalumoxane or fluorinated boranes which are cocatalysts of early transition metal catalysts for NBE polymerization usually react with polar monomers.18 Nickel-based catalysts have recently been used to catalyze the homopolymerization of polar norbornenes, but with low yields (less than 5%) unless an excess of Lewis acid cocatalyst relative to monomer is used.19–25 In 1992, Risse et al. demonstrated that [Pd(CH3CN)4][BF4]2 promoted the living polymerization of a series of esters of bicyclo[2.2.1]hept-5-ene-2-exo-methanol.26 Later, the same team reported that cationic (η3-allyl)palladium compounds with BF4− or SbF6− counter ions were able to catalyze the polymerization of bicyclo[2.2.1]hept-5-ene-2-carboxylic acid methyl ester (NBECO2Me).27 Copolymerization of NBE and bicyclo[2.2.1]hept-5-ene-2-carboxylic acid (NBECO2H) was also reported. In 1995, Novak et al. reported that neutral Pd(II) complexes bearing an hexafluoroacetyl acetonate and a σ–π bicyclic alkyl ligands catalyze the living polymerization of NBE and a substituted oxanorbornene.28 This catalyst, under its neutral form, is unable to catalyze the polymerization of NBECO2Me but a cationic analog was found to be active for the polymerization of NBECO2Me and the copolymerization of NBECO2H with NBE in a non-living manner, with either BF4−,29 SbF6− (ref. 30) or MAO30 as anion. These studies, and subsequent ones24,25,31–40 point out toward the same difficulties: (1) polar norbornenes are little reactive, and therefore the polymerization proceeds with low or moderate yield unless it is copolymerized (i.e. diluted) with NBE or with ethylene38,39,41 (2) resulting from the lack of reactivity of polar norbornenes, the polymerization must be performed with high catalyst loadings (>0.5 mol%); (3) the endo isomer is practically non-reactive and it deactivates the catalyst. The lack of reactivity of the endo substituted polar NBEs42,43 is believed to be due to the formation of a chelate between the Pd catalyst and the functionality either upon coordination of the monomer, or after insertion into the Pd–C bond. Although the insertion of NBE into Pd–C bonds is generally found to occur via the exo face,2,44–46 the formation of a chelate requires that the Pd atom adds to the endo face of the monomer, as shown by Sen et al. through the X-ray structure of an homologous Pt complex.42 At the opposite, Nozaki et al. has ruled out the formation of chelate for naked cationic Pd complexes stabilized by the tBu3P phosphine and found that endo isomers are as reactive as exo ones.47 Obviously, the mechanism of polymerization requires clarification.In this paper, we show that the lack of reactivity of the endo isomer is due to a series of factors (solubility, chelation and impossibility to insert two endo monomers consecutively). Furthermore, the catalyst promotes the rectification of the monomer (i.e. the interconversion from endo to exo, and vice versa). Numerous functional PNBEs (Scheme 1) have thus been prepared starting from monomers obtained directly from Diels–Alder reaction and not enriched in exo isomer, and using catalyst loadings as low as 0.002 mol%, which contrasts with literature data typically reported at catalyst loading of 1 mol%.
Results and discussion
Our initial work on the polymerization of polar norbornenes was inspired by the discovery that cationic ‘naked’ Pd and Ni catalysts2,27 are excellent catalysts for the homopolymerization of NBE. The term naked is used because the metal is believed to be only surrounded by the growing polymer chain, the monomer and/or solvent molecules with no ancillary ligand. These catalysts are cationic in nature, with a counter anion which belongs to the class of weakly coordinating anions.48 Several anions (BF4−, PF6−, MAO, B(C6F5)4−, SbF6−) have been investigated in the past for the polymerization of NBE, and we have selected SbF6− as it is more bulky than BF4− and PF6−, and therefore putatively less coordinating, but it does not require handling pyrophoric MAO and B(C6F5)3.We started our study with catalyst 1, [(η3-allyl)PdS2]+SbF6− (S = solvent).27 The polymerization of NBE(CO2Me) (73% endo) occurs in low to moderate yield (0–56%) at high catalyst loadings (≥0.2 mol%) in the majority of solvents (Table 1), in good agreement with past observations27 which have emphasized the difficulties associated with the polymerization of endo rich monomers. Surprisingly, in nitromethane, the polymerization proceeds with catalyst loadings as low as 0.02 mol%. Coordinating solvents such as DMSO, acetonitrile, acetone, water, DMF, methanol, ethyl acetate or THF efficiently solvate the ionic catalyst, but also inhibits monomer coordination (see Fig. 2 for catalyst structures coordinated by THF and water). Non-polar solvents such as tetrachloroethane, chlorobenzene or dichloromethane are only weakly (if at all) competing for the vacancy, resulting in yields which are higher, but the polymerization is then limited by the lack of solubility of the growing polymer chain: the polymerization eventually stops due to the precipitation of the cationic Pd active site anchored to the polymer, as visually observed. Nitromethane offers an acceptable compromise between catalytic deactivation via coordination and loss of solubility.
| Catalyst loading (mol%) | |||||||
|---|---|---|---|---|---|---|---|
| Solvent | 0.5 | 0.2 | 0.1 | 0.02 | 0.01 | 0.005 | 0.003 |
| Yielda (%) | |||||||
| a Monomer concentration = 2 g in 6.5 g solvent, 72 hours at room temperature. b T = 70 °C. | |||||||
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CH3CN | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acetone | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DMF | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CH3OH | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| THF | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Et2O | 36 | 16 | 0 | 0 | 0 | 0 | 0 |
| EtOAc | 73 | 18 | 0 | 0 | 0 | 0 | 0 |
| Toluene | 81 | 31 | 0 | 0 | 0 | 0 | 0 |
| C6H5Cl | 92 | 39 | 0 | 0 | 0 | 0 | 0 |
| DCM | 96 | 56 | 0 | 0 | 0 | 0 | 0 |
| CH3NO2 | 100 | 85 | 65 | 14 | 0 | 0 | 0 |
| Neat | 68 | 65 | 65 | 50b | 35b | 18b | 20b |
When the polymerization is performed neat (no solvent excepted traces of nitromethane during catalyst preparation), the yield is limited to 65% (catalyst loadings of 0.5–0.1%). This limitation is purely physical in nature, and corresponds to a vitrification phenomenon: a mixture of 65% of polymer and 35% of monomer is so viscous that the monomer cannot diffuse to reach the active site. Thus, the yield of the neat polymerization modestly decreases when the catalyst loading is lowered, so that the homopolymerization of NBE(CO2Me) becomes feasible even with a catalyst loading as low as 0.003 mol% which contrasts with past results reported in literature whereby catalyst loadings are typically comprised between 0.5 and 1%. In this study, all mechanistic studies have been performed in nitromethane as it keeps the polymer in solution without coordinating too strongly catalyst 1.
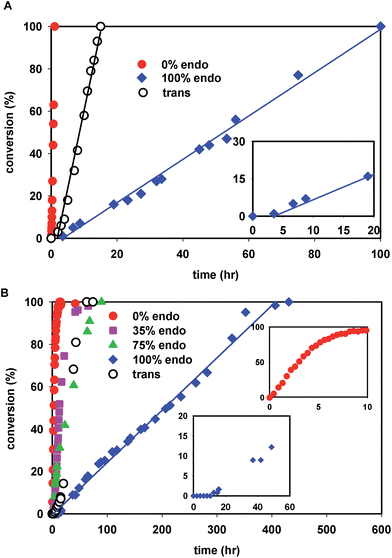
The kinetics of polymerization of cis-NBE(CO2Me)2 (abbreviated NBE(CO2Me)2) and trans-NBE(CO2Me)2 (one endo and one exo CO2Me group) are illustrated in Fig. 1. The rate of polymerization sharply decreases with increasing endo content, the kinetics of trans isomer being comprised between the one of 35% endo and of 75% endo, and therefore being comparable to 50% endo. When the endo content in the monomer is greater than 25%, the polymerization is zero order in monomer, as shown by a linear evolution of the conversion vs. time, a behavior which is also observed for the polymerization of other monomers with 1 (Fig. S35†). For the exo monomer, the kinetics deviates from zero order when [NBE(CO2Me)2]/[1] = 10 (Fig. 1B, inset). Furthermore, an induction period of a few hours is observed for the polymerization of the 100% endo monomer (Fig. 1A and B, insets).
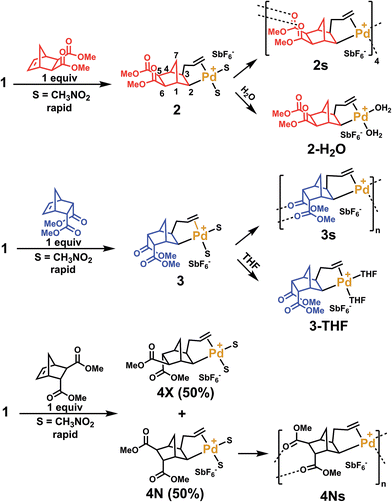
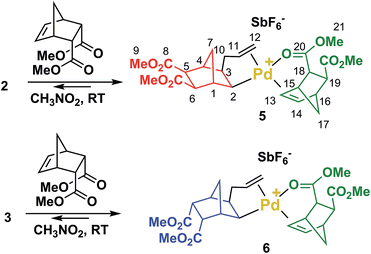
In order to clarify the mechanism of polymerization, exo, endo and trans NBE(CO2Me)2 were inserted in 1, leading to the formation of catalysts 2, 3 and 4 respectively (Scheme 2), which were fully characterized either by nuclear magnetic resonance (1H, 13C, DEPT, COSY and HMQC) and by X-ray crystallography (Fig. 2). The reaction of 1 with either endo, exo or trans NBE(CO2Me)2 is rapid, i.e. it is quantitative in less than a minute at room temperature (compared to several hours of polymerization, Fig. 1). The addition of the Pd–C bond to the double bond of NBE(CO2Me)2 occurs in a cis fashion on the exo face, as shown in X-ray structures. The 3J coupling value between protons H2 and H3 is comprised between 6 and 8 Hz which is characteristic of a cis coupling (see Scheme 2 for atom numbering). In NBE structures, 3J couplings between bridgehead protons and protons in endo positions are less than 2 Hz, whereas couplings with protons in exo positions are of the order of 3–4 Hz.49 No 3J coupling between protons H1 and H2 or H3 and H4 could be observed in our case, which is consistent with a Pd attack on the exo face of the monomer. It has been proposed in the past that catalyst addition could occur on the endo face of endo-substituted monomers, because of the directing effect of the functional group.42 In our case, coupling patterns of all Pd species characterized in this work (3J between protons H1 and H2 or H3 and H4) are consistent with an exo placement of the Pd atom, ruling out the presence of a directing effect. Addition of NBE(CO2Me)2 (trans) to 1 results in the formation of two products (4X and 4N) in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mol% ratio, as shown by 1H NMR (Fig. S11†). In the 4X (resp. 4N) product, the Pd atom is on the same side of the bridge as the exo (resp endo) CO2Me. The fact that 4X and 4N are in equal proportion is another indication that the endo ester does not act as a directing group for catalyst addition. In solution, the ester groups of the inserted NBE(CO2Me)2 are not coordinated, as shown by 13C NMR chemical shifts comprised between 173.2 and 175.5 ppm for C
50 mol% ratio, as shown by 1H NMR (Fig. S11†). In the 4X (resp. 4N) product, the Pd atom is on the same side of the bridge as the exo (resp endo) CO2Me. The fact that 4X and 4N are in equal proportion is another indication that the endo ester does not act as a directing group for catalyst addition. In solution, the ester groups of the inserted NBE(CO2Me)2 are not coordinated, as shown by 13C NMR chemical shifts comprised between 173.2 and 175.5 ppm for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of complexes 2, 3, 4N and 4X, which correspond to the usual chemical shifts of CO2Me esters. For the sake of comparison, in a norbornane ring bearing two CO2Me groups in exo (resp trans), the C
O of complexes 2, 3, 4N and 4X, which correspond to the usual chemical shifts of CO2Me esters. For the sake of comparison, in a norbornane ring bearing two CO2Me groups in exo (resp trans), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O resonates at 173.1 ppm (ref. 50) (resp 173.6 and 174.6 ppm)51 whereas coordinated CO2Me either on these naked Pd complexes (see below) or on cationic Pd diimine complexes are found at lower field (177–195 ppm).52
O resonates at 173.1 ppm (ref. 50) (resp 173.6 and 174.6 ppm)51 whereas coordinated CO2Me either on these naked Pd complexes (see below) or on cationic Pd diimine complexes are found at lower field (177–195 ppm).52
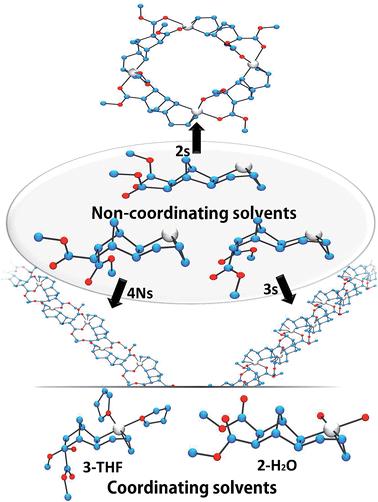
Crystals of complexes 2, 3 and 4N were formed by adding a non-coordinating solvent and/or by letting the solvent evaporate slowly (the letter s stands for solid-state structure). Structure 2s is a tetrameric macrocycle whereas 3s and 4Ns are polymeric (Fig. 2). In these solid-state structures, intermolecular coordination of the ester is observed, however, when the crystallization experiment is performed in the presence of a stronger Lewis base, such as water and THF, monomeric complexes (2-H2O and 3-THF) are obtained. The tetrameric solid 2s is soluble in non-coordinating solvents such as dichloromethane, chlorobenzene and tetrachloroethane however, polymeric 3s and 4Ns are insoluble in such solvents. Therefore, when the polymerization is performed in a non-coordinating solvent, the insertion of endo and the trans monomer leads to the formation of an insoluble active species (3s and 4s). Thus, the lack of reactivity of endo isomers in chlorinated solvents is in part explainable by the loss of Pd-containing species by precipitation. Interestingly, characteristic bond lengths and bond angles of complexes 2s, 3s, 4Ns, 2-H2O and 3-THF (Table S1†) are quite similar, once again pointing out that there is no significant structural difference for the first insertion product of the endo and exo monomers. However kinetic plots in nitromethane (Fig. 1) point out toward a very different reactivity for endo and exo isomers. Thus differences must occur after the first monomer insertion.
The reaction of 2 or 3 with 1 equivalent of endo-NBE(CO2Me)2 at room temperature result in the immediate formation of 5 and 6 (Scheme 3), which were fully characterized by NMR (1H, 13C, DEPT, COSY and HMQC). In these complexes, endo-NBE(CO2Me)2 is chelated via its endo face to the Pd complex, as deduced by an unexpected downfield resonance for the HC13![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C14H moiety (1H NMR: 7.02 for 5, 7.22 ppm for 6vs. 6.29 ppm for the uncoordinated monomer and 13C NMR: 135.7 for 5 and 135.4 ppm for 6vs. 134.7 ppm for the uncoordinated monomer). The 13C carbonyl resonances are also shifted downfield (177.5 and 177.1 respectively for 5 and 6vs. 172.7 ppm for the uncoordinated monomer). This chelate is labile and rapidly exchanging within NMR timescale. For example, from 7.05 ppm in 5, the H13/14 resonance is displaced to 6.83 ppm for a 1
C14H moiety (1H NMR: 7.02 for 5, 7.22 ppm for 6vs. 6.29 ppm for the uncoordinated monomer and 13C NMR: 135.7 for 5 and 135.4 ppm for 6vs. 134.7 ppm for the uncoordinated monomer). The 13C carbonyl resonances are also shifted downfield (177.5 and 177.1 respectively for 5 and 6vs. 172.7 ppm for the uncoordinated monomer). This chelate is labile and rapidly exchanging within NMR timescale. For example, from 7.05 ppm in 5, the H13/14 resonance is displaced to 6.83 ppm for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5 and endo-NBE(CO2Me)2, and to 6.48 ppm for a 1
1 mixture of 5 and endo-NBE(CO2Me)2, and to 6.48 ppm for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mixture of 5 and endo-NBE(CO2Me)2. Such exchange phenomenon is not observed when an excess of exo monomer is added to 5 or 6 as shown by separate resonances for the chelated endo monomer and free exo-NBE(CO2Me)2 (Fig. S26 and S28†). Therefore, if it occurs, displacement of the chelated endo monomer by the exo monomer is slow.
10 mixture of 5 and endo-NBE(CO2Me)2. Such exchange phenomenon is not observed when an excess of exo monomer is added to 5 or 6 as shown by separate resonances for the chelated endo monomer and free exo-NBE(CO2Me)2 (Fig. S26 and S28†). Therefore, if it occurs, displacement of the chelated endo monomer by the exo monomer is slow.
When 2 is reacted with 10 equivalents of exo-NBE(CO2Me)2, polymerization is quantitative within 9 hours, as shown by the decrease of the olefin resonances in 1H NMR at 6.35 ppm. To our surprise, although no endo monomer was added to this reaction, the presence of the chelated complex 5 is also clearly detected in the reaction mixture, as shown by the presence of (1) a new downfield olefin resonance (6.8–7.0 ppm) characteristic of the endo chelated double bond, (2) a OCH3 resonance at 3.81 ppm which is neither observed in 2 (3.74 ppm) nor in the exo growing polymer chain (3.74 ppm) and (3) a characteristic H17 bridge proton for the chelated endo monomer at 1.71 ppm (Fig. S26†). The exo monomer used in this study contained traces of endo monomer (less than 2%) which could account for a maximum of 20% of the chelated Pd complexes (10 equivalents of monomer were used relative to Pd). However, as much as 70% of the Pd atoms are found to be chelated. Therefore, the endo isomer must be generated during the polymerization reaction. Lewis acids are known to catalyze direct and retro Diels–Alder reactions,53 and therefore Lewis acidic cationic Pd complexes could be responsible for the transformation of the exo monomer in endo monomer. Although is it well known that the exo monomer (thermodynamic product) is more stable than the endo monomer (kinetic product), DFT calculations indicate that the gain of stability is only 2.7 kJ mol−1. Therefore, the equilibrium constant between both isomers is 3, and the exo–endo monomer distribution at equilibrium is 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. Thus, even when starting from 100% exo monomer, endo isomer is generated during the polymerization. It will be seen below that the reverse situation is also true, that is to say that when starting from pure endo monomer, exo monomer is generated during the reaction.
25. Thus, even when starting from 100% exo monomer, endo isomer is generated during the polymerization. It will be seen below that the reverse situation is also true, that is to say that when starting from pure endo monomer, exo monomer is generated during the reaction.
When the exo monomer is polymerized by 2, the Lewis-acid catalyzed formation of the endo isomer leads to the generation of chelated 5 which is significantly less active for polymerization. Thus, only a fraction of the catalyst is 'naked' and active, resulting in the formation of polymers with molecular weights which are higher than expected (Table 2, entry 5, 10, 15–17). The higher than expected molecular weights may also originate from the low initiating-ability of catalyst 2. The initiation of the polymerization of exo-NBE(CO2Me)2 by 2 (rate constant ki) is slow relative to subsequent insertions (kp) as the Pd–C2–C3–C10–C11–C12 6-member chelate must be broken during the first insertion. When polymerizing endo-NBE(CO2Me)2, the propagation rate (kp) is greatly decreased, thus the discrepancy between kp and ki is less noticeable. As the result, experimental and theoretical molecular weights are in good agreement for endo monomers (Table 2, entry 1, 6, 11). The in situ formation of chelated 5 during the polymerization of exo-NBE(CO2Me)2 explains the deviation from linearity observed in the polymerization kinetics at high conversion (see inset Fig. 1B). Initially, all the active sites are unchelated, and the polymerization proceeds rapidly. As the polymerization progresses, the endo isomer is generated in situ, leading to the gradual formation of chelated catalysts which are less active, and resulting in a decrease of the polymerization rate. Such effect is not observed when polymerizing endo-NBE(CO2Me)2 or mixtures of endo and exo NBE(CO2Me)2, as the catalyst is then entirely chelated from the onset of the polymerization.
| Expt | Endo (%) | Cat loading mol% | Yield/% | M n,theor g mol−1 | M n g mol−1 | PDIa |
|---|---|---|---|---|---|---|
| a Number average molecular weight and polydispersity index determined by GPC-LS in THF. b Without ( ): 24 hours reaction, with ( ): 72 hours reaction. | ||||||
| 1 | 100 | 1 | 40 (59)b | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.3 |
| 2 | 75 | 1 | 83 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.5 |
| 3 | Trans | 1 | 92 (99) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
| 4 | 35 | 1 | 98 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.9 |
| 5 | 0 | 1 | 100 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
241![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.2 |
| 6 | 100 | 0.2 | 15 (21) | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
| 7 | 75 | 0.2 | 56 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.5 |
| 8 | Trans | 0.2 | 55 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.6 |
| 9 | 35 | 0.2 | 85 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.6 |
| 10 | 0 | 0.2 | 100 | 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
380![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
| 11 | 100 | 0.1 | 8 (10) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.4 |
| 12 | 75 | 0.1 | 37 (39) | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.5 |
| 13 | Trans | 0.1 | 16 (42) | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
| 14 | 35 | 0.1 | 65 (69) | 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.4 |
| 15 | 0 | 0.1 | 100 | 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
389![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.5 |
| 16 | 0 | 0.02 | (13) | 136![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
509![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.2 |
| 17 | 0 | 0.01 | (4) | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
316![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.4 |
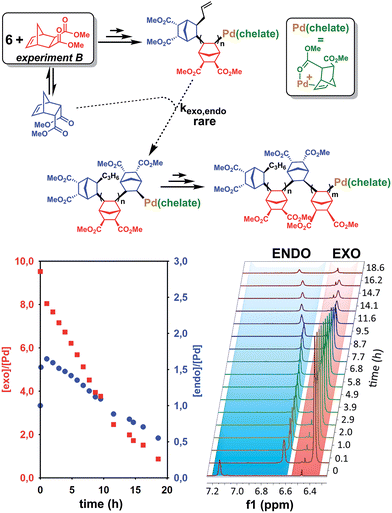
The last paragraph pertained to the polymerization of exo-NBE(CO2Me)2. However, it is desirable to polymerize monomers directly obtained by Diels–Alder reaction, that is to say rich in endo isomer in order to avoid a painstaking separation step between endo and exo isomers. In this case, due to the presence of excess endo isomer, the catalyst is entirely chelated. Therefore, we will now concentrate on the reactivity of catalysts 5 and 6. Using 1H NMR, one can assess the regiochemistry of the last inserted monomer unit. Indeed, the methine proton in α of Pd (H2, Scheme 3) resonates respectively at 4.3 and 3.9 ppm when it is part of an endo and an exo unit. When 6 is reacted with 10 equivalents of endo-NBE(CO2Me)2 (experiment A in Scheme 4), polymerization occurs very slowly over a period of 500 hours. During the first 9 hours, no polymerization is observed (corresponding to an induction period, see inset Fig. 1B). By contrast, when 6 is reacted with 10 equivalents of exo-NBE(CO2Me)2 (experiment B), insertion of a first exo monomer occurs within minutes (Fig. S28†). Thus, the addition of an exo monomer after an endo unit is very rapid (experiment B), whereas the addition of two consecutive endo units is very slow (experiment A). Using the usual denomination for copolymerization rate constants, kendo,exo ≫ kendo,endo. The value of kendo,exo is too high to be measured precisely via1H NMR, but a lower limit for kendo,exo could be determined on the account that the insertion of one exo monomer by 6 proceeds in less than 10 minutes (Fig. S28 and S32†), that is to say a minimum of 6 insertions per hour, thus, kendo,exo ≥ 6 h−1. The isomerization of exo-NBE(CO2Me)2 in endo-NBE(CO2Me)2 is clearly observed in experiment B (Fig. 3). At the beginning of the polymerization, the only endo monomer present is engaged in the chelate 6 (1 equivalent relative to Pd), but soon after, 1.6 equivalents of endo monomer are present (Fig. 3). Despite the presence of a significant amount of endo monomer, its insertion is never observed (no endo H2 protons, Fig. S28†). This could either be due to the fact that the endo monomer is non-reactive (it is not inserted), or that the insertion of endo monomer is immediately followed by the insertion of an exo monomer, due to the high value of kendo,exo. To lift this ambiguity, we have examined the reaction of 5 with 10 equivalents of endo-NBE(CO2Me)2 (experiment C in Scheme 4, Fig. S29†).
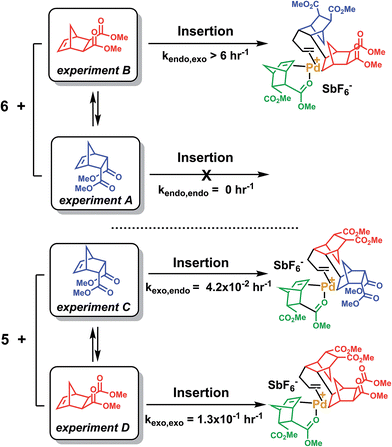 | ||
| Scheme 4 Reaction of NBE(CO2Me)2 with 5 or 6. Only the first insertion is shown for the sake of simplification. | ||
In experiment C, endo-NBE(CO2Me)2 is inserted, as shown by the apparition over a few hours of a characteristic doublet at 4.3 ppm corresponding to H2 in an endo unit. Thus, the endo monomer is reactive and kexo,endo is non null. The low stability of catalyst 5 in solution is another indication that the endo isomer can be inserted after an exo unit. Indeed, when a solution of 5 in CD3NO2 ([5] = 0.032 mol L−1) is left for 7 hours, 30% of the chelated endo monomer is inserted (Fig. S30†). To determine the value of kexo,endo, the initial rate at which the endo monomer is inserted into the Pd-exo-C2 bond has been measured in three separate experiments (experiment C performed with respectively 0.5, 1 and 7 equivalents of endo NBE(CO2Me)2, Fig. S33†). The reactions are zero order with respect to monomer concentration (as shown by linear kinetic profiles, Fig. S33†), probably because the rate determining step is the insertion of the coordinated monomer in the Pd–C bond. The values of kexo,endo, obtained from the slope of the kinetic profiles, are respectively 3.6 × 10−2, 4.1 × 10−2 and 4.9 × 10−2 h−1, which yield an average value of kexo,endo of 4.2 × 10−2 h−1 (Table 3).
| Rate constant, h−1 | Reactivity ratio | |
|---|---|---|
| k exo,exo | 1.3 × 10−1 | r exo = 3.1 |
| k exo,endo | 4.2 × 10−2 | |
| k endo,exo | >6 | r endo = 0 |
| k endo,endo | ∼0 | |
Contrasting with the lack of stability of 5 in solution (insertion of the endo chelate in Pd-exo-C2), solutions of 6 are stable indefinitely (Fig. S31†), indicating that kendo,endo ∼0. Therefore, two endo units cannot be inserted consecutively. The presence of an induction period for experiment A can be explained by the necessity for the catalyst to interconvert some of the endo-NBE(CO2Me)2 into exo-NBE(CO2Me)2 before polymerization can proceed. The value of kexo,exo was measured in two separate kinetic experiments (experiment D in Scheme 4 conducted with respectively 3 and 10 equivalents of exo monomer, Fig. S34†), leading to kexo,exo = 0.13 h−1. Once again, the kinetics is zero order with respect to monomer concentration. From the determination of the rate constants and reactivity ratios (Table 3), it is clear that the polymerization of functional polar norbornenes present some unique features. As rendo = 0, an endo unit is always isolated in the chain. When polymerizing an exo monomer, endo monomers are generated via retro Diels–Alder reaction and inserted as isolated units within the mainly exo chain (Scheme 5). When an endo monomer is polymerized, an exo monomer generated by retro Diels–Alder reaction is immediately inserted due to the high value of kendo,exo; therefore the monomer feed is nearly exclusively constituted of endo monomer. Although the addition of an exo monomer after an exo unit is 3.1 times faster than the addition of an endo monomer (rexo = 3.1), the insertion of two exo monomers consecutively is highly unlikely due to the low concentration of exo monomer in solution. Thus, the polymerization of the endo monomer leads to the formation of an alternating endo–exo copolymer. The catalyst has rectified 50% of the less reactive endo monomers into more reactive exo monomers. We have coined such mechanism rectification–insertion.
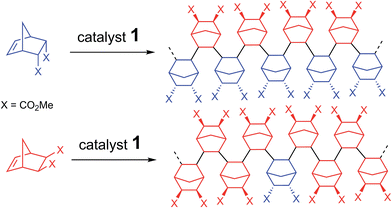 | ||
| Scheme 5 Polymerization via the rectification–insertion mechanism of functional PNBEs prepared from respectively endo and exo monomers. | ||
Microstructural analysis of these polymers, either by 1H NMR or 13C NMR has been hampered by the broadness of the peaks caused by the rigidity of polynorbornenes in solution (Fig. S37–48†). For the polymer prepared with exo NBE(CO2Me)2, the 13C spectrum is constituted of very broad resonances, with a single peak observed at 173.1 ppm for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Fig. S37–38†). The complete absence of fine structure is consistent with the presence in the polymer of exo and endo units statistically distributed. In contrast, for the polymer prepared with the endo monomer, the 1H and 13C NMR spectra are constituted of slightly sharper peaks, which are consistent with the regioregular structure of the alternating polymer. Three resonances observed at 172.4, 173.4 and 174.3 ppm in a ∼2
O (Fig. S37–38†). The complete absence of fine structure is consistent with the presence in the polymer of exo and endo units statistically distributed. In contrast, for the polymer prepared with the endo monomer, the 1H and 13C NMR spectra are constituted of slightly sharper peaks, which are consistent with the regioregular structure of the alternating polymer. Three resonances observed at 172.4, 173.4 and 174.3 ppm in a ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio respectively (Fig. S39†) could conceivably correspond to one resonance for endo C
1 ratio respectively (Fig. S39†) could conceivably correspond to one resonance for endo C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and two resonances for exo C
O and two resonances for exo C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (endo C
O (endo C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are upfield relative to exo ones in 2, 3, 5 and 6). The presence of two exo C
O are upfield relative to exo ones in 2, 3, 5 and 6). The presence of two exo C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks in same proportion could arise from two different tacticities arising from the placement of consecutive NBEs.
O peaks in same proportion could arise from two different tacticities arising from the placement of consecutive NBEs.
In order the rectification–insertion mechanism to be operating, two conditions must be met. First, the endo isomer must be interconverted into exo. It is also well established that Lewis acids (such as 1) catalyze the Diels–Alder and retro Diels–Alder reaction.53–55 Since all the monomers of this study (Table 4) have been prepared by Diels–Alder reaction in one step, such interconversion is expected, albeit it is anticipated to be slower for poor dienophiles such as allyl alcohol. In the case of NBE(CHO), the occurrence of retro Diels–Alder reaction during the polymerization is revealed by the presence of inserted dicyclopentadiene within the polymer (acroleine loss by evaporation). We also have checked that dicyclopentadiene can be homopolymerized by 1 (yield = 30% for 0.2 mol% catalyst loading at 70 °C). Interestingly, freshly cracked cyclopentadiene can also be polymerized with 1, leading to a polymer which is not entirely similar to polydicyclopentadiene by 1H NMR (precise analysis of this polymer is beyond the scope of this paper, as it not a norbornene polymer). Second, kendo,endo must be significantly lower than the other propagation rate constants. Preliminary theoretical calculations indicate that kendo,endo is very low because of the large steric hindrance between the endo substituents of the penultimate inserted unit and the active site when two endo monomers are inserted in a row. Several elements point toward the fact the rectification–insertion mechanism is not only prevailing with NBE(CO2Me)2 but also with other functionalized norbornenes. Induction periods are observed for the polymerization of predominantly endo monomers (Fig. S35†), whereas induction periods are not observed for predominantly exo monomers. This induction period corresponds to the time period necessary to rectify the endo isomer into the exo isomer in order to unblock the endo-terminated growing chain. Furthermore, the 13C NMR spectra of polymers prepared with mostly endo monomer or mostly exo monomer are not clearly different, and contain overlapping resonances, which is consistent with polymers which are constituted of both endo and exo units. With the rectification–insertion mechanism in operation, endo isomers become polymerizable. Thus, by extension, the polymerization can proceed with monomers containing as much as 70–100% endo isomer, that is to say, monomers obtained directly from Diels–Alder reaction which are not enriched in exo isomer (Table 4).
| Monomer | Endo (%) | Cat. loading (mol%) | Yield/% | M n g mol−1 | PDIc |
|---|---|---|---|---|---|
| a In solution in CH3NO2. b Without solvent. c Determined by GPC (see ESI for conditions). | |||||
| NBE(CO2Me)a | 73 | 0.1 | 65 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.2 |
| NBE(CO2Me)b | 73 | 0.01 | 35 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.5 |
| NBE(CO2H)a | 75 | 0.1 | 71 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.2 |
| NBE(CO2H)b | 75 | 0.1 | 57 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.2 |
| NBE(CO2H)b | 75 | 0.01 | 40 | 252![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
| NBE(CO2H)b | 45 | 0.1 | 55 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.7 |
| NBE(CO2H)b | 45 | 0.01 | 40 | 477![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.1 |
| NBE(CO2H)b | 45 | 0.002 | 15 | 609![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.6 |
| NBE(CO2H)2a | 0 | 1 | 93 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.5 |
| CAa | 0 | 0.5 | 83 | 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.1 |
| CAa | 0 | 0.2 | 13 | 315![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
| NBE(CH2Br)b | 86 | 0.2 | 15 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.2 |
| NBE(imide)a | 35 | 1 | 80 | 8050 | 1.3 |
| NBE(imide)a | 35 | 0.1 | 45 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.6 |
| NBE(imide)a | 5 | 1 | 80 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.1 |
| NBE(CH2OH)b | 82 | 0.2 | 53 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
| NBE(CHO)b | 80 | 0.2 | 73 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.6 |
| NBE(CHO)b | 80 | 0.02 | 21 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.9 |
Thanks to this mechanism, catalyst 1 proved to be active for a wide array of monomers (Tables 1, 2 and 4). The catalyst is very versatile, as it is able to function in the presence of alcohols, esters, carboxylic acids, anhydrides, aldehydes, alkyl bromides, and amides. To our knowledge, out of the nine monomers probed here, four had never been polymerized before (CA, NBE(CO2H)2, NBE(imide), and NBE(CHO)). As a proof of the unique versatility of this reaction, the polymerization of aldehyde containing monomer NBE(CHO) was found to proceed in high yield (73% at 0.2 mol% catalyst loading) which contrasts with radical, cationic and anionic polymerizations which are usually not efficient to prepare linear polymers containing pendant aldehyde groups.56 Furthermore, this method is not only efficient for organosoluble but also for water soluble polymers such as PNBE(CO2H)2.
When the polymerization is performed in the absence of solvent, very low amounts of catalyst can be used (as low as 0.002 mol%), which is indicative of the exceptional robustness of the active species. The polymerization is then controlled by the drastic increase of viscosity associated with the formation of high Tg polymers, a physical limitation which could for example be mitigated by the use of heterophase processes. The polymers have in general low polydispersity indices (1.1 ≤ PDI ≤ 1.6), indicating some degree of livingness for this type of polymerization (a feature which will be further explored in a subsequent report). Monomers with high exo content (PNBE(imide) 5% endo, PCA, PNBE(CO2H)2) lead to polymers with a molecular weight higher than expected. As shown above, this behavior is a consequence of the rectification–insertion mechanism: even when starting from pure exo monomer, endo chelated species can be formed, and only the remaining (unchelated) fraction is rapidly polymerizing. When the monomer contains high amount of endo isomer, experimental molecular weights are commensurate with theoretical values, which is again indicative of a high degree of control for the polymerization. All these polymers exhibit Tg which are higher than 300 °C (Fig. S48†), which is a consequence of the high rigidity of the PNBE backbone. Finally, it should be noted that the polymerization is highly tolerant, as monomers could even be polymerized in air (catalyst 1 was prepared under nitrogen) with virtually identical yields to those obtained under inert atmosphere. For example, the polymerization of NBE(CO2H) (75% endo, catalyst = 0.01 mol%, 24 hours at 70 °C) occurs in 40% and 42% yield when performed respectively under inert atmosphere and in air.
Conclusions
The novel rectification–insertion polymerization mechanism is a powerful mechanism for the preparation of rigid macromolecules obtained from polar NBEs, yielding functional polymers bearing highly valuable functional groups such as aldehydes, anhydrides, alcohols, alkyl halogens and carboxylic acids. Thus, this method offers the same level of versatility and practicality as highly popular chain-growth polymerizations such as ROMP or radical polymerizations. The reaction readily proceeds with endo-rich monomers directly obtained via Diels–Alder reaction with no need for cumbersome and time-consuming separation of both isomers. Furthermore, catalyst loadings as low as 0.002 mol% can be used, and both the monomer preparation and the polymerization can be performed in the absence of any solvent. Thus, the preparation of these rigid macromolecules is an archetypal example of green chemical process. This study has also aimed at clarifying the mechanism of polymerization of substituted NBEs. The endo isomers deactivate the catalyst because the endo active species are less soluble than the exo ones and the endo monomer forms a chelate with the naked catalyst. However, these limitations can be counteracted by the judicious choice of polymerization conditions, and, most importantly, by the action of the rectification–insertion mechanism. Thus, the naked Pd complex has a tandem role of polymerization catalyst and of endo/exo isomerization catalyst. As two endo units cannot be inserted consecutively, it is possible to prepare alternating endo–exo copolymers when starting from an endo monomer only. We envision that this novel mechanism could be easily exploited further, for example by adding a separate Lewis acid which could catalyze the retro/direct Diels–Alder reaction, which should putatively lead to a rate acceleration and the disappearance of the induction period. Furthermore, due to high degree of control of these polymerization, we believe that this mechanism open the way to the formation of a wealth of hierarchical nanostructures generated upon self-assembly of rigid functional amphiphilic block PNBEs.Acknowledgements
This work was supported by the NanoQuebec program (industry cooperation), the company Microbonds and NSERC (Discovery grant). We thank Moubarak Campaore, Florian Pierre, Alexandre Arnold, Jonathan Potier and Vladimir Kriuchkov for expert assistance and Prof A. Soldera (University of Sherbrooke, Canada) for DFT calculations. We also thank Dr G. Yap and Mr G. Andrade (University of Delaware) for X-Ray crystallographic analysis.Notes and references
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- B. Goodall, in Late Transition Metal Polymerization Catalysis, ed. B. Rieger, L. S. Baugh, S. Kacker and S. Striegler, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, FRG, 2003, pp. 101–154 Search PubMed.
- R. Ma, Y. Hou, J. Gao and F. Bao, Polym. Rev., 2009, 49, 249–287 CrossRef CAS.
- A. Buono, A. Famulari, S. V. Meille, G. Ricci and L. Porri, Macromolecules, 2011, 44, 3681–3684 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed.
- N. J. Warren, O. O. Mykhaylyk, D. Mahmood, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 1023–1033 CrossRef CAS PubMed.
- G. Gody, C. Rossner, J. Moraes, P. Vana, T. Maschmeyer and S. Perrier, J. Am. Chem. Soc., 2012, 134, 12596–12603 CrossRef CAS PubMed.
- N. Zhang, S. Salzinger, B. S. Soller and B. Rieger, J. Am. Chem. Soc., 2013, 135, 8810–8813 CrossRef CAS PubMed.
- N. Zhang, S. Salzinger, F. Deubel, R. Jordan and B. Rieger, J. Am. Chem. Soc., 2012, 134, 7333–7336 CrossRef CAS PubMed.
- N. M. G. Franssen, J. N. H. Reek and B. de Bruin, Chem. Soc. Rev., 2013, 42, 5809–5832 RSC.
- C. W. Bielawski and R. H. Grubbs, Prog. Polym. Sci., 2007, 32, 1–29 CrossRef CAS PubMed.
- R. Khurana, J. L. Schaefer, L. A. Archer and G. W. Coates, J. Am. Chem. Soc., 2014, 136, 7395–7402 CrossRef CAS PubMed.
- G. Sun, S. Cho, C. Clark, S. V. Verkhoturov, M. J. Eller, A. Li, A. Pavía-Jiménez, E. A. Schweikert, J. W. Thackeray, P. Trefonas and K. L. Wooley, J. Am. Chem. Soc., 2013, 135, 4203–4206 CrossRef CAS PubMed.
- D. Moatsou, C. F. Hansell and R. K. O'Reilly, Chem. Sci., 2014, 5, 2246 RSC.
- W. Kaminsky, L. Boggioni and I. Tritto, in Polymer Science: A Comprehensive Reference, ed. K. Matyjaszewski and M. Moller, Elsevier, Amsterdam, 2012, pp. 843–873 Search PubMed.
- L. Boggioni and I. Tritto, MRS Bull., 2013, 38, 245–251 CrossRef CAS.
- E. Y.-X. Chen, Chem. Rev., 2009, 109, 5157–5214 CrossRef CAS PubMed.
- F. Blank and C. Janiak, Coord. Chem. Rev., 2009, 253, 827–861 CrossRef CAS PubMed.
- P. Huo, W. Liu, X. He, H. Wang and Y. Chen, Organometallics, 2013, 32, 2291–2299 CrossRef CAS.
- Y. Xing, Y. Chen and X. He, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4425–4432 CrossRef CAS.
- X. He, Y. Liu, L. Chen, Y. Chen and D. Chen, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4695–4704 CrossRef CAS.
- S. Martínez-Arranz, A. C. Albéniz and P. Espinet, Macromolecules, 2010, 43, 7482–7487 CrossRef.
- J. A. Casares, P. Espinet, J. M. Martín-Alvarez, J. M. Martínez-Ilarduya and G. Salas, Eur. J. Inorg. Chem., 2005, 2005, 3825–3831 CrossRef.
- S. Kaita, K. Matsushita, M. Tobita, Y. Maruyama and Y. Wakatsuki, Macromol. Rapid Commun., 2006, 27, 1752–1756 CrossRef CAS.
- C. Shikada, S. Kaita, Y. Maruyama, M. Takei and Y. Wakatsuki, Macromol. Rapid Commun., 2008, 29, 219–223 CrossRef CAS.
- S. Breunig and W. Risse, Die Makromol. Chemie, 1992, 193, 2915–2927 CrossRef CAS.
- J. P. Mathew, A. Reinmuth, J. Melia, N. Swords and W. Risse, Macromolecules, 1996, 29, 2755–2763 CrossRef CAS.
- A. L. Safir and B. M. Novak, Macromolecules, 1995, 28, 5396–5398 CrossRef CAS.
- B. Heinz, W. Heitz and S. Krügel, Acta Polym., 1997, 48, 385–391 CrossRef CAS.
- B. S. Heinz, F. P. Alt and W. Heitz, Macromol. Rapid Commun., 1998, 19, 251–256 CAS.
- U. Okoroanyanwu, T. Shimokawa, J. Byers and C. G. Willson, Chem. Mater., 1998, 10, 3319–3327 CrossRef CAS.
- I. G. Jung, J. Seo, Y. K. Chung, D. M. Shin, S.-H. Chun and S. U. Son, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3042–3052 CrossRef CAS.
- T. Saito and Y. Wakatsuki, Polymer, 2012, 53, 308–315 CrossRef CAS PubMed.
- B.-G. Shin, M.-S. Jang, D. Y. Yoon and W. Heitz, Macromol. Rapid Commun., 2004, 25, 728–732 CrossRef CAS.
- B.-G. Shin, T.-Y. Cho, D. Y. Yoon and B. Liu, Macromol. Res., 2013, 15, 185–190 CrossRef.
- L. Y. Wang, Y. F. Li, F. M. Zhu and Q. Wu, Eur. Polym. J., 2006, 42, 322–327 CrossRef CAS PubMed.
- F. He, Y. Chen, X. He, M. Chen, W. Zhou and Q. Wu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3990–4000 CrossRef CAS.
- S. Liu, S. Borkar, D. Newsham, H. Yennawar and A. Sen, Organometallics, 2007, 26, 210–216 CrossRef CAS.
- A. Ravasio, L. Boggioni and I. Tritto, Macromolecules, 2011, 4180–4186 CrossRef CAS.
- K. Müller, Y. Jung, D. Y. Yoon, S. Agarwal and A. Greiner, Macromol. Chem. Phys., 2010, 211, 1595–1601 CrossRef.
- B. M. Boardman and G. C. Bazan, Acc. Chem. Res., 2009, 42, 1597–1606 CrossRef CAS PubMed.
- A. D. Hennis, J. D. Polley, G. S. Long, A. Sen, D. Yandulov, J. Lipian, G. M. Benedikt, L. F. Rhodes and J. Huffman, Organometallics, 2001, 20, 2802–2812 CrossRef CAS.
- J. K. Funk, C. E. Andes and A. Sen, Organometallics, 2004, 23, 1680–1683 CrossRef CAS.
- J. G. P. Delis, P. G. Aubel, K. Vrieze, P. W. N. M. van Leeuwen, N. Veldman and A. L. Spek, Organometallics, 1997, 16, 4150–4160 CrossRef CAS.
- B. A. Markies, D. Kruis, M. H. P. Rietveld, K. A. N. Verkerk, J. Boersma, H. Kooijman, M. T. Lakin, A. L. Spek and G. van Koten, J. Am. Chem. Soc., 1995, 117, 5263–5274 CrossRef CAS.
- M. D. Walter, R. A. Moorhouse, S. A. Urbin, P. S. White and M. Brookhart, J. Am. Chem. Soc., 2009, 131, 9055–9069 CrossRef CAS PubMed.
- I. Takamiya, M. Yamashita and K. Nozaki, Organometallics, 2008, 27, 5347–5352 CrossRef CAS.
- I. Krossing and I. Raabe, Angew. Chem., Int. Ed., 2004, 43, 2066–2090 CrossRef CAS PubMed.
- E. Ban, R. P. Hughes and J. Powell, J. Organomet. Chem., 1974, 69, 455–472 CrossRef CAS.
- D. E. James and J. K. Stille, J. Org. Chem., 1976, 41, 1504–1511 CrossRef CAS.
- S. Niwayama, H. Cho, M. Zabet-Moghaddam and B. R. Whittlesey, J. Org. Chem., 2010, 75, 3775–3780 CrossRef CAS PubMed.
- L. K. Johnson, S. Mecking and M. Brookhart, J. Am. Chem. Soc., 1996, 118, 267–268 CrossRef CAS.
- P. A. Grieco and N. Abood, J. Org. Chem., 1989, 54, 6008–6010 CrossRef CAS.
- E. J. Corey, Angew. Chem., Int. Ed., 2002, 41, 1650–1667 CrossRef CAS.
- Z. Pi and S. Li, J. Phys. Chem. A, 2006, 110, 9225–9230 CrossRef CAS PubMed.
- M. Delgado, M. Desroches and F. Ganachaud, RSC Adv., 2013, 3, 23057–23065 RSC.
Footnote |
| † Electronic supplementary information (ESI): Experimental procedures, NMR characterization, kinetic plots, ORTEP diagrams and cif files. CCDC 1034345–1034348 and 1034422. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc03575e |
| This journal is © The Royal Society of Chemistry 2015 |