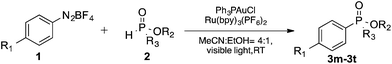
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A dual catalytic strategy for carbon–phosphorus cross-coupling via gold and photoredox catalysis†
Ying
He
ab,
Hongmiao
Wu
ab and
F. Dean
Toste
*b
aInstitute of Chemistry and BioMedical Sciences, Nanjing University, Nanjing, 210046, China
bDepartment of Chemistry, University of California, Berkeley, CA 94720, USA. E-mail: fdtoste@berkeley.edu
First published on 25th November 2014
Abstract
A new method for the P-arylation of aryldiazonium salts with H-phosphonates via dual gold and photoredox catalysis is described. The reaction proceeds smoothly at room temperature in the absence of base and/or additives, and offers an efficient approach to arylphosphonates. The reaction is proposed to proceed through a photoredox-promoted generation of an electrophilic arylgold(III) intermediate that undergoes coupling with the H-phosphonate nucleophile.
Introduction
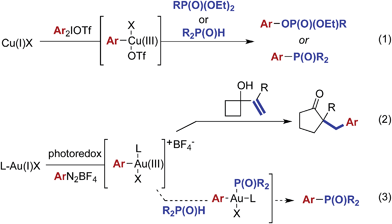
During the past decade, homogeneous gold reactions based on Au(I) or Au(III) catalysis have emerged as an extraordinary tool to create molecular complexity. In these reactions, gold most-commonly acts as a redox-neutral and carbophilic π-acid that activates carbon–carbon multiple bonds towards nucleophilic attack.1 Alternatively, gold-catalyzed transformations employing a stoichiometric external oxidant, such as Selectfluor, have allowed entry into pathways involving Au(I)/Au(III);2 however, the majority of these reactions still involve intermediates generated from activation of a carbon–carbon π-bond. The requirement for stoichiometric amounts of strong oxidizing reagents has generally limited the chemistry to π-bonds and aromatic compounds.3 Recently, stepwise oxidation of gold(I) complexes by photoredox-generated4 radical species has emerged as an alternative strategy for accessing Au(I)/Au(III) coupling reactions.5Organophosphorus compounds have drawn increasing attention due to their broad applications in biological, pharmaceutical, and material sciences.6 These compounds are commonly accessed through transition metal-catalyzed coupling processes.7 More recently, the desired coupling has been achieved through the reaction of phosphonate esters8 or phosphine oxides9 with highly electrophilic arylcopper(III) intermediates10 generated from oxidation of copper(I) with diaryliodionium(III) salts [eqn (1)]. While the reported combined photoredox/gold-catalyzed reactions have relied on the intervention of carbon–carbon π-bonds [eqn (2)], we hypothesized that the gold(III) intermediates generated in this manner might also engage in coupling reactions with other nucleophilic species [eqn (3)].11
Results and discussions
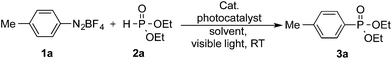
To this end, we explored the dual photoredox/gold-catalyzed coupling reaction of p-tolyldiazonium12 with diethyl phosphite (Table 1). The initial screening of solvents found that the desired product was formed in 37% yield when acetonitrile was employed as solvent (Table 1, entry 1). Other solvents commonly employed in photocatalysis, such as DMF and EtOH, were also tested and afforded the product in 50% and 65%, respectively (Table 1, entries 2 and 3). In order to exploit the better solubility of diazonium salts and tautomerization of H-phosphonates13 in polar solvents, we explored whether a solvent mixture with ethanol might improve the reaction outcome. The co-solvent consisting of MeCN/EtOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave the best result, affording the arylphosphonate in 82% yield (Table 1, entries 4–7). Lower yields were obtained when Ru(bpy)3Cl2 or Ir(ppy)3 were used as the photocatalyst (Table 1, entries 8 and 9). No product was observed with IPrAuCl as the gold catalyst (Table 1, entry 10), and reducing the amount of photocatalyst or gold catalyst both gave lower yields (Table 1, entries 11 and 12). Moreover, no product was detected when the reaction was performed in the absence of the gold catalyst (Table 1, entry 13) and significantly reduced yields of 3a were observed in the absence of the Ru(bpy)3(PF6)2 and/or light (Table 1, entries 14 and 15). We also examined replacing the gold catalyst with those derived from palladium, silver or copper salts; however, only moderate yield was obtained when Pd(OAc)2 was used (Table 1, entry 16), and no product was detected with other catalysts even when the reaction time was prolonged to 16 h (Table 1, entries 17–22).
1) gave the best result, affording the arylphosphonate in 82% yield (Table 1, entries 4–7). Lower yields were obtained when Ru(bpy)3Cl2 or Ir(ppy)3 were used as the photocatalyst (Table 1, entries 8 and 9). No product was observed with IPrAuCl as the gold catalyst (Table 1, entry 10), and reducing the amount of photocatalyst or gold catalyst both gave lower yields (Table 1, entries 11 and 12). Moreover, no product was detected when the reaction was performed in the absence of the gold catalyst (Table 1, entry 13) and significantly reduced yields of 3a were observed in the absence of the Ru(bpy)3(PF6)2 and/or light (Table 1, entries 14 and 15). We also examined replacing the gold catalyst with those derived from palladium, silver or copper salts; however, only moderate yield was obtained when Pd(OAc)2 was used (Table 1, entry 16), and no product was detected with other catalysts even when the reaction time was prolonged to 16 h (Table 1, entries 17–22).
| Entry | Cat. | Photocatalyst | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions were carried out at room temperature with a 26 W household bulb, 1a (0.3 mmol), 2a (0.1 mmol), cat. (10 mol%), photocatalyst (2 mol%), degassed solvent (0.5 ml), N2 atmosphere, rt. b Isolated yields. c 1 mol% Ru(bpy)3(PF6)2 was used. d 5 mol% Ph3PAuCl was used. e Reaction run in the dark. f 10 mol% PPh3 was used as the ligand. | |||||
| 1 | Ph3PAuCl | Ru(bpy)3(PF6)2 | MeCN | 4 | 37 |
| 2 | Ph3PAuCl | Ru(bpy)3(PF6)2 | DMF | 4 | 50 |
| 3 | Ph3PAuCl | Ru(bpy)3(PF6)2 | EtOH | 4 | 65 |
| 4 | Ph3PAuCl | Ru(bpy)3(PF6)2 | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 49 |
| 5 | Ph3PAuCl | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 82 |
| 6 | Ph3PAuCl | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 1 EtOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 61 |
| 7 | Ph3PAuCl | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 9 EtOH = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 58 |
| 8 | Ph3PAuCl | Ru(bpy)3Cl2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 77 |
| 9 | Ph3PAuCl | Ir(ppy)3 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 24 |
| 10 | IPrAuCl | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 0 |
| 11c | Ph3PAuCl | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 73 |
| 12d | Ph3PAuCl | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 54 |
| 13 | — | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 0 |
| 14 | Ph3PAuCl | — | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | <10 |
| 15e | Ph3PAuCl | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | <5 |
| 16 | Pd(OAc)2 | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 43 |
| 17 | AgNTf2 | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 | 0 |
| 18 | AgBF4 | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 | 0 |
| 19f | AgBF4 | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 | 0 |
| 20 | AgOTf | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 | 0 |
| 21 | Cu(OAc)2 | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 | 0 |
| 22 | CuI | Ru(bpy)3(PF6)2 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 4 EtOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 | 0 |
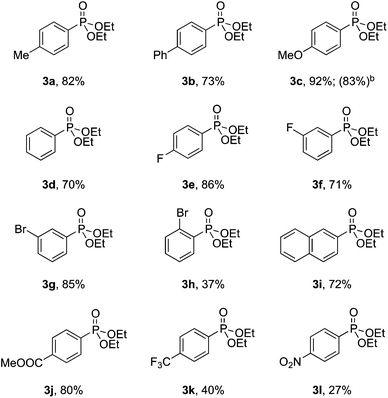
With the optimized conditions in hand, we investigated the scope of the diazonium substrates. Aryldiazoniums salts bearing electron-donating groups at their para-positions, such as methyl, phenyl and methoxy, were coupled with diethyl phosphite affording the corresponding products in good to excellent yields (Table 2, compounds 3a–3c). However, the reactivity was dramatically decreased when aryldiazoniums salts containing strong withdrawing groups such as –CF3 and –NO2 were used (Table 2, compounds 3k and 3l). As expected, the P-arylation using aryl diazoniums with halogen in their para and meta positions proceeded efficiently, and yields of 71–86% were obtained (Table 2, compounds 3e–3g). However, 2-bromophenyl diazonium was less reactive comparatively and 37% yield of the product was obtained (Table 2, compound 3h). The naphthyl phosphonate was isolated in 72% yield under the standard reaction conditions (Table 2, compound 3i).
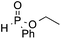
We next turned our attention to an evaluation of the scope and limitations of our reaction with different types of P(O)H compounds. As seen in Table 3, aryl diazonium salts with electron-donating, electron-withdrawing and halogen substituents reacted with H-phosphonate diesters bearing different alkyl groups efficiently, and yields of 74–90% were obtained (Table 3, entries 1–5). The reaction also proceeded smoothly with dibenzyl and ethyl phenylphosphinate as coupling partners (Table 3, entries 6 and 8). The more challenging coupling of H-phosphonate diphenylester also occurred under the gold-catalyzed reaction conditions, albeit it in diminished yield (Table 3, entry 7).
| Entry | R1 | P(O)H compounds | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 1 (0.3 mmol), 2 (0.1 mmol), Ph3PAuCl (10 mol%), Ru(bpy)3(PF6)2 (2 mol%), degassed MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = (4 EtOH = (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.5 ml), N2 atmosphere, visible light, rt. for 4h.
b Isolated yield.
c MeCN 1) (0.5 ml), N2 atmosphere, visible light, rt. for 4h.
b Isolated yield.
c MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = (4 MeOH = (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.5 ml) as the solvent.
d MeCN 1) (0.5 ml) as the solvent.
d MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) iPrOH = (4 iPrOH = (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.5 ml) as the solvent. 1) (0.5 ml) as the solvent.
|
|||
| 1c | OMe |
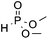
|
3m, 90 |
| 2c | F |
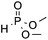
|
3n, 74 |
| 3c | COOMe |
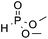
|
3o, 88 |
| 4d | OMe |
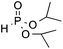
|
3p, 81 |
| 5d | F |
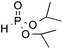
|
3q, 84 |
| 6 | OMe |
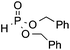
|
3r, 50 |
| 7 | OMe |
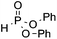
|
3s, 31 |
| 8 | OMe |
|
3t, 82 |
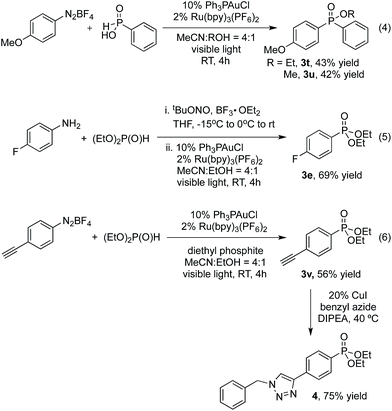
Interestingly, phenyl phosphinic acid was also a competent nucleophile for this reaction yielding products of a three-component coupling between the diazonium salt, arylphosphinic acid and the alkyl alcohol solvent [eqn (4)].
Additionally, the intermediate aryl diazonium salt can be generated without purification from the corresponding aniline. For example, 3e was obtained in 69% through one-pot, two-step procedure for the diazotization and P-arylation of 4-fluoroaniline, compared with 86% under standard conditions [eqn (5)].
Conclusions
In conclusion, we have developed the first gold-catalyzed oxidative P-arylation of H-phosphonates promoted by visible light photoredox catalysis. The reaction proceeds under mild reaction conditions (room temperature, no base) and shows excellent substrate scope, including the use of phosphinic acids as coupling partners. More broadly, the use of photoredox catalysis to achieve the oxidation event required for cross-coupling,5,14 avoids the need for strong oxidants associated with known gold-catalyzed coupling reactions.15 This feature putatively allows for increased functional group compatibility, as clearly demonstrated by the gold-catalyzed formation of alkyne-substituted phosphinate ester 3v, in which the potentially reactive carbon–carbon π-bond16 is left intact, and can subsequently be engaged in a copper-catalyzed alkyne-azide click reaction17 [eqn (6)]. The development of this strategy for cross-coupling and detailed mechanistic studies is ongoing in our group.Acknowledgements
We are grateful for financial support from the NIHGMS (RO1 GM073932), the National Natural Science Foundation of China (21332005), and Jiangsu Educational Innovation Team Program (P.R. China). We also thank Matthew S. Winston, Mark D. Levin, David A. Nagib and Miles W. Johnson for helpful discussions.Notes and references
- For selected reviews: (a) N. D. Shapiro and F. D. Toste, Synlett, 2010, 675–691 CAS; (b) A. Fürstner, Chem. Soc. Rev., 2009, 38, 3208–3221 RSC; (c) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351–3378 CrossRef CAS PubMed; (d) D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395–403 CrossRef CAS PubMed; (e) M. N. Hopkinson, A. D. Gee and V. Gouverneur, Chem.–Eur. J., 2011, 17, 8248–8262 CrossRef CAS PubMed; (f) H. A. Wegner and M. Auzias, Angew. Chem., Int. Ed., 2011, 50, 8236–8247 CrossRef CAS PubMed; (g) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef PubMed.
- (a) G. Z. Zhang, Y. Peng, L. Cui and L. M. Zhang, Angew. Chem., Int. Ed., 2009, 48, 3112–3115 CrossRef CAS PubMed; (b) G. Z. Zhang, L. Cui, Y. Z. Wang and L. M. Zhang, J. Am. Chem. Soc., 2010, 132, 1474–1475 CrossRef CAS PubMed; (c) W. E. Brenzovich, D. Benitez, A. D. Lackner, H. P. Shunatona, E. Tkatchouk, W. A. Goddard and F. D. Toste, Angew. Chem., Int. Ed., 2010, 49, 5519–5522 CrossRef PubMed; (d) A. D. Melhado, W. E. Brenzovich, A. D. Lackner and F. D. Toste, J. Am. Chem. Soc., 2010, 132, 8885–8886 CrossRef CAS PubMed; (e) N. P. Mankad and F. D. Toste, J. Am. Chem. Soc., 2010, 132, 12859–12861 CrossRef CAS PubMed; (f) M. N. Hopkinson, A. Tessier, A. Salisbury, G. T. Giuffedi, L. E. Combettes, A. D. Gee and V. Gouverneur, Chem.–Eur. J., 2010, 16, 4739–4743 CrossRef CAS PubMed; (g) W. E. Brenzovich, J. F. Brazeau and F. D. Toste, Org. Lett., 2010, 12, 4728–4731 CrossRef CAS PubMed; (h) L. T. Ball, M. Green, G. C. Lloyd-Jones and C. A. Russel, Org. Lett., 2010, 12, 4724–4727 CrossRef CAS PubMed; (i) M. N. Hopkinson, J. E. Ross, G. T. Giuffredi, A. D. Gee and V. Gouverneur, Org. Lett., 2010, 12, 4904–4907 CrossRef CAS PubMed; (j) W. Wang, J. Jasinski, G. B. Hammond and B. Xu, Angew. Chem., Int. Ed., 2010, 49, 7247–7252 CrossRef CAS PubMed; (k) T. de Haro and C. Nevado, Angew. Chem., Int. Ed., 2011, 50, 906–910 CrossRef CAS PubMed; (l) G. Z. Zhang, Y. D. Luo, Y. Z. Wang and L. M. Zhang, Angew. Chem., Int. Ed., 2011, 50, 4450–4454 CrossRef CAS PubMed; (m) A. Leyva-Pérez, A. Doménech, S. I. Al-Resayes and A. Corma, ACS Catal., 2012, 2, 121–126 CrossRef; (n) A. Arcadi, E. Pietropaolo, A. Alvino and V. Michelet, Org. Lett., 2013, 15, 2766–2769 CrossRef CAS PubMed; (o) R. Zhang, Q. Xu, K. Chen, P. Gu and M. Shi, Eur. J. Org. Chem., 2013, 2013, 7366–7371 CrossRef CAS; (p) For an approach using iodine(III) as an oxidant see: L. T. Ball, G. C. Lloyd-Jones and C. A. Russell, Chem.–Eur. J., 2012, 18, 2931–2937 CrossRef CAS PubMed.
- (a) J. P. Brand, J. Charpentier and J. Waser, Angew. Chem., Int. Ed., 2009, 48, 9346–9349 CrossRef CAS PubMed; (b) T. de Haro and C. Nevado, J. Am. Chem. Soc., 2010, 132, 1512–1513 CrossRef CAS PubMed; (c) L. T. Ball, G. C. Lloyd-Jones and C. A. Russell, Science, 2012, 337, 1644–1648 CrossRef CAS PubMed; (d) J. P. Brand and J. Waser, Org. Lett., 2012, 14, 744–747 CrossRef CAS PubMed; (e) L. T. Ball, G. C. Lloyd-Jones and C. A. Russell, J. Am. Chem. Soc., 2014, 136, 254–264 CrossRef CAS PubMed.
- For reviews on visible-light photoredox catalysis, see: (a) K. Zeitler, Angew. Chem., Int. Ed., 2009, 48, 9785–9789 CrossRef CAS PubMed; (b) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed; (c) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (d) M. A. Ischay and T. P. Yoon, Eur. J. Org. Chem., 2012, 2012, 3359–3372 CrossRef CAS; (e) J. W. Tucker and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 1617–1622 CrossRef CAS PubMed; (f) J. Xuan and W. J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828–6838 CrossRef CAS PubMed; (g) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (h) Y. M. Xi, H. Yi and A. W. Lei, Org. Biomol. Chem., 2013, 11, 2387–2403 RSC; (i) M. N. Hopkinson, B. Sahoo, J. L. Li and F. Glorius, Chem.–Eur. J., 2014, 20, 3874–3886 CrossRef CAS PubMed.
- (a) B. Sahoo, M. N. Hopkinson and F. Glorius, J. Am. Chem. Soc., 2013, 135, 5505–5508 CrossRef CAS PubMed; (b) X. Z. Shu, M. Zhang, Y. He, H. Frei and F. D. Toste, J. Am. Chem. Soc., 2014, 136, 5844–5847 CrossRef CAS PubMed; (c) M. S. Winston, W. J. Wolf and F. D. Toste, J. Am. Chem. Soc., 2014, 136, 7777–7782 CrossRef CAS PubMed.
- For selected reviews: (a) A. Skarżyńska, Coord. Chem. Rev., 2013, 257, 1039–1048 CrossRef PubMed; (b) J. L. Montchamp, Acc. Chem. Res., 2014, 47, 77–87 CrossRef CAS PubMed; (c) C. Queffélec, M. Petit, P. Janvier, D. A. Knight and B. Bujoli, Chem. Rev., 2012, 112, 3777–3807 CrossRef PubMed; (d) C. S. Demmer, N. Krogsgaard-Larsen and L. Bunch, Chem. Rev., 2011, 111, 7981–8006 CrossRef CAS PubMed; (e) L. Kollár and G. Keglevich, Chem. Rev., 2010, 110, 4257–4302 CrossRef PubMed; (f) S. Van der Jeught and C. V. Stevens, Chem. Rev., 2009, 109, 2672–2702 CrossRef CAS PubMed; (g) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029–3069 CrossRef CAS PubMed.
- For recent examples, see: (a) G. Hu, W. Chen, T. Fu, Z. Peng, H. Qiao, Y. Gao and Y. Zhao, Org. Lett., 2013, 15, 5362–5365 CrossRef CAS PubMed; (b) O. Berger, C. Petit, E. L. Deal and J. L. Montchamp, Adv. Synth. Catal., 2013, 355, 1361–1373 CrossRef CAS; (c) K. Xu, H. Hu, F. Yang and Y. Wu, Eur. J. Org. Chem., 2013, 2013, 319–325 CrossRef CAS; (d) A. J. Bloomfield and S. B. Herzon, Org. Lett., 2012, 14, 4370–4373 CrossRef CAS PubMed; (e) S. M. Rummelt, M. Ranocchiari and J. A. van Bokhoven, Org. Lett., 2012, 14, 2188–2190 CrossRef CAS PubMed; (f) C. R. Shen, G. Q. Yang and W. B. Zhang, Org. Biomol. Chem., 2012, 10, 3500–3505 RSC; (g) E. L. Deal, C. Petit and J. L. Montchamp, Org. Lett., 2011, 13, 3270–3273 CrossRef CAS PubMed.
- M. Fañanás-Mastral and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 9894–9897 CrossRef PubMed.
- J. Xu, P. B. Zhang, Y. Z. Gao, Y. Y. Chen, G. Tang and Y. F. Zhao, J. Org. Chem., 2013, 78, 8176–8183 CrossRef CAS PubMed.
- (a) A. J. Walkinshaw, W. Xu, M. G. Suero and M. J. Gaunt, J. Am. Chem. Soc., 2013, 135, 12532–12535 CrossRef CAS PubMed; (b) M. G. Suero, E. D. Bayle, B. S. L. Collins and M. J. Gaunt, J. Am. Chem. Soc., 2013, 135, 5332–5335 CrossRef CAS PubMed.
- We have previously observed C–P reductive elimination in the formation of tertaarylphosphonium salt by-products of the dual photoredox/gold-catalyzed process reported in ref. 5b.
- For recent reviews on the reactions of aryldiazonium salts, see: (a) D. P. Hari and B. König, Angew. Chem., Int. Ed., 2013, 52, 4734–4743 CrossRef CAS PubMed; (b) F. Y. Mo, G. B. Dong, Y. Zhang and J. B. Wang, Org. Biomol. Chem., 2013, 11, 1582–1593 RSC.
- J. Stawinski and A. Kraszewski, Acc. Chem. Res., 2002, 35, 952–960 CrossRef CAS PubMed.
- (a) D. Kalyani, K. B. McMurtrey, S. R. Neufeldt and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 18566–18569 CrossRef CAS PubMed; (b) Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. C. MacMillan, Science, 2014, 345, 437–440 CrossRef CAS PubMed; (c) J. C. Tellis, D. N. Primer and G. A. Molander, Science, 2014, 345, 433–436 CrossRef CAS PubMed.
- M. D. Levin and F. D. Toste, Angew. Chem., Int. Ed., 2014, 53, 6211–6215 CrossRef CAS PubMed.
- Carbon–carbon π-bonds as reactive towards the previously employed selectfluor oxidant. For example, the Ag-catalyzed phosphonofluorination of π-bonds: C. Zhang, Z. Li, L. Zhu, L. Yu, Z. Wang and C. Li, J. Am. Chem. Soc., 2013, 135, 14082–14085 CrossRef CAS PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, full experimental details and characterisation. See DOI: 10.1039/c4sc03092c |
| This journal is © The Royal Society of Chemistry 2015 |